Bortezomib-induced cutaneous lesions in multiple myeloma patients: A case report
Published Web Location
https://doi.org/10.5070/D30cv3t3f4Main Content
Bortezomib-induced cutaneous lesions in multiple myeloma patients: A case report
M Rodríguez-Martín1, M Sáez-Rodríguez1, M García-Bustínduy1, A Martín-Herrera2, A Noda-Cabrera1
Dermatology Online Journal 14 (11): 14
1. Department of Dermatology2. Department of Pathology
Hospital Universitario de Canarias, University of La Laguna, 38320 - La Laguna, Tenerife, Spain. marinarm@gmail.com
Abstract
Proteasome inhibitors are emerging as a promising class of anti-cancer therapeutic agents. Bortezomib (PS341) is the first proteosome inhibitor with clinical significance. It acts by blocking vital functions of tumoral cells in myeloma, inducing apoptosis. Its toxicity is usually manageable. Gastrointestinal symptoms, peripheral neuropathy, neuropathic pain and thrombocytopenia are described as the most common side effects. We report on a case of cutaneous lesions induced by bortezomib in a patient with relapsed multiple myeloma (MM).
Case
A 65-year-old man was referred to our Department for the development of asymptomatic nodules and a purpuric rash on the trunk, back and neck. His medical history included refractory MM (Durie-Salmon stage IIIB, IgA kappa) diagnosed one year prior to presentation and ischemic cardiopathy.
He had received vincristine, adriamycin, dexamethasone, melphalan, cyclophosphamide, and autologous hematopoietic stem cell transplantation as previous treatments due to refractary MM. Finally, intravenous bolus injection of bortezomib (1.3 mg/meter² of body surface area) were administered as monotherapy twice weekly for two weeks followed by the same regimen after a 10-day rest period (bortezomib is administered on days 1, 4, 8 and 11, every 21 days).
Cutaneous lesions appeared after the sixth administration of bortezomib during the second treatment cycle (day 25). No fever, pain, nor itching was observed with the onset of cutaneous lesions. No changes in previous treatments (folic acid, omeprazol, acetylsalicylic acid) had been made for the past month except for the addition of bortezomib. No other toxicity symptoms potentially related to bortezomib were reported.
 |
| Figure 1 |
|---|
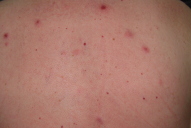
| Figure 1. Purpuric rash on chest, back and neck and multiple erythematous, sharply demarcated, papulo-nodular lesions, from 0.5 to 1.5 cm in diameter could be observed. |
Physical examination revealed a purpuric rash on chest, back and neck and multiple erythematous, sharply demarcated, papulo-nodular lesions, from 0.5 to 1.5 cm in diameter (Fig. 1). The rest of the physical examination was normal.
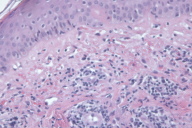
A cutaneous biopsy specimen was taken. Histological examination revealed perivascular lymphoid infiltrates with nuclear dust, erythrocyte extravasation and discrete collagen necrosis. Minimal spongiosis was present and apoptotic keratinocytes were also found (Fig. 2).
With these clinical and histological findings, diagnosis of drug-induced cutaneous eruption was made.
Cutaneous lesions resolved after 7 days of treatment with 30 mg of oral prednisone per day. Bortezomib was not discontinued in our patient. Prednisone 10 mg was prophylactically administered before each bortezomib infusion and no recurrence of the cutaneous lesions were observed.
Multiple myeloma (MM) is a hematologic neoplasm characterized by the monoclonal proliferation of bone marrow plasma cells [1]. Several advances have been made in the treatment of MM over the past decade, especially with the arrival of new, active agents such as thalidomide (Thalomid®), bortezomib (Velcade®), and lenalidomide (Revlimid®) [1, 2]. These have shown significant clinical activity as single agents.
Bortezomib is the first of a new class of medications called proteasome inhibitors. It is a modified dipeptidyl boronic acid that reduces the NF-κB translocation/transcription activity and blocks drug-related signalling pathways critical to vital functions of myeloma cells [1]. This drug has also been shown to inhibit the adherence between bone marrow stroma and myeloma cells, to block apoptosis resistance, and to inhibit IL-6 induced proliferation and angiogenesis [1, 2].
Proteosome enzyme complex degrades about 80 percent of all inner cellular proteins, so an overdosage of bortezomib could be lethal. Bortezomib has shown to be effective in refractory and relapsed MM. Phase-3 trials demonstrated that bortezomib is better for treating relapse MM than high dose dexamethasone [3] as a single agent. In addition, there is evidence that in the treatment of myeloma patients, bortezomib in combination with corticosteroids is more effective than bortezomib alone. Therefore, the first choice for MM treatment is the combination of these two drugs [3].
In most of the cases, bortezomib-associated rash is asymptomatic and it is clinically characterized by the presence of multiple erythematous nodules or plaques located on the trunk, back, and neck. However, a great variety of clinical pictures of bortezomib-induced cutaneous adverse reactions have been described, such as morbiliform exanthems and ulcerations on the trunk, erythematous nodules on the trunk, face and neck, erythematous papules on the chest and neck, erythematous plaques on the trunk and limbs, leucoclastic vasculitis, Sweet syndrome, purpura, or an eruption covered with a black eschar on the nose [2, 4, 5, 6].
Typically, the bortezomib-induced eruption appears after at least 2 cycles of this drug. However, cutaneous lesions have been described during the first, second, third, and fourth treatment cycles [2, 3, 4, 5, 6]. Only one patient in the literature had to discontinue treatment because of the severity of the eruption and the lack of response [2]. Most cases in the literature have shown a good response with low-dose corticosteroids prophylactically before each administration of bortezomib. Antihistamines are generally ineffective treatments [2, 4, 5, 6, 7].
Bortezomib administration may enhance the release of pro-inflammatory cytokines (including IL-6 and TNF-α) and the generation of a cell-mediated immune response, which could play a role in several bortezomib-induced cutaneous reactions [2, 4]. A recent report suggests that development of a rash is a possible surrogate marker of a good response of non-Hodgkin lymphoma to bortezomib [8].
Therapy with proteosome inhibitors is an increasing practice in medicine. As a result, cutaneous reactions will probably be noted more frequently. Therefore, our recommendation is that cutaneous biopsy should be done to support clinical diagnosis of drug-related skin eruption. Early recognition of cutaneous adverse reactions during bortezomib therapy is extremely important because treatment with corticosteroids have proven to be effective and allows the continuation of the drug.
References
1. Mateos MV, San Miguel J. Effect of bortezomib in multiple myeloma. Best Pract Res Clin Haematol. 2007 Dec; 20(4):701-15. Review. PubMed2. Lung-Wu K, Heule F, Lam K, Sonneveld P. Pleomorphic presentation of cutaneous lesions associated with the proteasome inhibitor bortezomib in patients with multiple myeloma. J Am Acad Dermatol 2006; 55: 897-900. PubMed
3. Nozza A, Siracusano L, Armando S. Bortezomib-dexamethasone combination in a patient with refractory multiple myeloma and impaired renal function. Clin Ther. 2006 Jun; 28(6):953-9. PubMed
4. Min C-K, Lee S, Kim Y-J, Eom K-S et al. Cutaneous leucoclastic vasculitis (LV) following bortezomib therapy in a myeloma patient; association with pro-inflamatory cytokines. Eur J Haematol 2006; 76: 265-268. PubMed.
5. Knoops L, Jacquemain A, Tennsted D, Theate I, et al. Bortezomib-induced Sweet syndrome. Br J Haematol 2005; 131:142. PubMed
6. Agterof MJ, Biesma DH. Images in clinical medicine: bortezomib-induced skin lesions. N Engl J Med 2005; 16: 2534. PubMed
7. Ali Ozcan M, Alacacioglu I, Piskin O, Lebe B, et al. Bortezomib-Induced skin lesions. Acta Haematol 2006; 116: 226-227. PubMed
8. Gerecitano J, Goy A, McGregor Cortelli B, Neylon E, et al. Drug-induced cutaneous vasculitis in patients with non-Hodgkin's lymphoma receiving bortezomib: a possible surrogate marker of response. Br J Haematol. 2006 Aug; 134(4):391-8. PubMed
© 2008 Dermatology Online Journal