Generalized granuloma annulare resolving to anetoderma
Published Web Location
https://doi.org/10.5070/D308z9j753Main Content
Generalized granuloma annulare resolving to anetoderma
Ummuhan Kiremitci1, Serife Karagulle1, Elif Topcu1, Mehmet Salih Gurel1, Sila Seremet Erdogan1, Asli Turgut Erdemir1, Alper Alyanak1, Cuyan Demirkesen2
Dermatology Online Journal 12 (7): 16
1. Dermatology Clinic, Istanbul Training and Research Hospital, Istanbul, TURKEY2. Department of Pathology, Istanbul University,
Cerrahpasa Medical Faculty, Istanbul, TURKEY
Abstract
Granuloma annulare is a benign, degenerative skin disease; the generalized form is rare. The question of an association between generalized granuloma annulare and other internal diseases has been a matter of debate for many years. We report a 54-year-old latent-diabetic patient with generalized granuloma annulare who developed anetoderma during its treatment.
Clinical synopsis
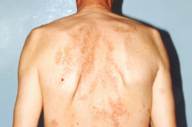
|
| Figure 1 |
|---|
| Erythematous papules in annular distribution |
A 54-year-old man presented with a 2-year history of multiple erythematous asymptomatic lesions on the trunk and extremities. Dermatologic examination revealed multiple 1-5-mm skin-colored or bright yellow-red papules on the neck, trunk, and extensor surfaces of the extremities (Fig. 1). Annular lesions were also present. A general physical examination was normal. His medical and family history was insignificant. Laboratory tests, including CBC, ESR, urinalysis, and biochemical analyses, were within the normal range. Although his fasting glucose concentration was normal, the 2-hour value of blood sugar following 75-g oral glucose tolerance test was 200mg/dl and HbA1c was 6.2 percent; these results were accepted as signs of latent diabetes mellitus. Chest x-ray, ultrasonography of abdomen, thyroid functional tests, the levels of immunoglobulin and complements were normal. Serological tests were negative. HbsAg and anti HCV were negative but anti-HBs antibodies were positive. He had not been vaccinated against hepatitis B virus.
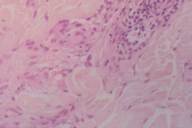
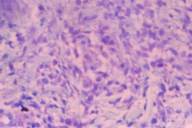
Histopathological evaluation of biopsy specimens revealed loss of rete ridges in epidermis, a mild increase of pigmentation in basal layer, and dense eosinophilic histiocytes between the collagen fibers. Also observed were fragmented elastic fibers in the cytoplasm of some histiocytes, perivascular inflammatory infiltrate consisting of mononuclear cells and few eosinophils (Fig. 2). Mucopolysaccharide deposition between degenerated collagen bundles was demonstrated with alcian blue stain (Fig. 3). These findings supported the diagnosis of GA.
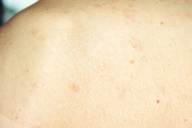
|
| Figure 4 |
|---|
| Atrophic scars on the shoulder |
PUVA treatment, 4 times a week, was administered for 3 months and then it was stopped cause he returned to his home in the country. After PUVA treatment, he was treated with dapsone (200 mg daily). Topical corticosteroid was applied to the lesions during the therapy. The patient was evaluated monthly. The majority of the lesions have completely healed leaving hypo- and hyper-pigmented atrophic areas (Fig. 4). The punch biopsy of the atrophic lesion revealed a mild atrophy in epidermis and homogenization in the connective tissue of superficial dermis. These findings were consistent with anetoderma. Finally clinical and histopathological findings of our case were diagnosed as a generalized granuloma annulare healed with anetoderma.
Discussion
Granuloma annulare (GA) has varying clinical presentations that include localized, generalized, subcutaneous, and perforating forms [1]. A generalized form of granuloma annulare (GGA) can occur in up to 15 percent of patients, and it is characterized by hundreds-to-thousands of small, symmetric, asymptomatic, erythematous or skin-colored 1-2 mm papules, especially located on neck, trunk and extensor surfaces of extremities [2]. Generalized granuloma annulare typically has a later onset of disease, poorer response to therapy, and an increased prevalence of the HLA-Bw35 allele [2, 3].
Diabetes and serum lipid abnormalities are more common in patients with generalized GA as compared to the other forms of GA [1, 4, 5]. There is a twofold increase in the incidence of diabetes mellitus in GA patients compared with the general population [1, 6, 7].
An association between GA and diabetes mellitus was reported in 12 percent of the GA patients. Studer found three latent, four type-I, and three type-II diabetics among 84 GA patients [8]. Our patient was determined to have latent diabetes mellitus. In addition, multiple lesions of GA in children are associated with significantly lower serum insulin values than in controls and mildly impaired glucose tolerance [9]. Diabetic patients suffer significantly more often from chronic relapsing GA than nondiabetic patients [8].
Generalized granuloma annulare rarely resolves spontaneously and shows a poor therapeutic response. These circumstances have led to the use of numerous treatments, including systemic corticosteroids, systemic retinoids, chloroquine, potassium iodide, dapsone, niacinamide, chlorpropamide, chlorambucil, topical tacrolimus, systemic PUVA therapy, PUVA bath therapy, and UVA1 phototherapy [7, 10]. In our case GGA lesions healed leaving atrophic areas following treatment with topical corticosteroids and PUVA for 3 months followed by dapsone for 3 months. The diagnosis of anetoderma was established after histological evaluation which revealed epidermal atrophy and homogenization of the connective tissue of superficial dermis. The giant cells may engulf elastic tissues in generalized granuloma annulare, therefore secondary anetoderma may be an expected outcome after generalized granuloma annulare [11].
Anetoderma is characterized clinically by small, atrophic papules that herniate inward at palpation and histopathologically by loss of dermal elastic tissue [12].
Anetoderma is termed primary when it occurs on normal skin without an underlying disease or dermatoses and secondary when lesions appear at the site of inflammatory dermatoses. Dermatoses reported to resolve with anetoderm include lupus erythematosus, sarcoidosis, acne, leprosy, pilomatricoma, prurigo nodularis, cutaneous plasmacytoma, benign cutaneous lymphoid hyperplasia, urticaria pigmentosa, perifolliculitis, syphilis, tuberculosis, xanthoma, nodular amyloidosis, thyroidal disorders, varicella, juvenile xanthogranuloma, and granuloma annulare [11, 12, 13].
The pathologic mechanisms of anetoderma are unknown. Anetoderma may result from an imbalance between metalloproteinases and inhibitors of metalloproteinases in lesional skin, resulting in degradation of elastic tissue [12, 13, 14]. It has been suggested that human macrophage metalloelastase may contribute to elastin degradation or phagocytosis of elastic tissue by macrophage can play role in development of anetoderma after granuloma annulare lesions [11].
Although there are numerous studies on GA, we have found only one case report in the dermatological literature of GGA as a precursor of anetoderma [11]. Ozkan reported a 33-year-old woman with anetoderma secondary to GGA; she had 0.5-2.0 cm hypopigmented atrophic papules and plaques appearing on sun-exposed areas of her body for 10 years. Individual lesions of GGA had transformed to anetodermic lesions within 5 to 6 months [11]. Our case had smaller and more numerous lesions; anetoderma developed during treatment and as early as 3 months. We conclude that generalized granuloma annulare is one of the causes of secondary anetoderma.
References
1. Lynch JM, Barrett TL. Collagenolytic (necrobiotic) granulomas: part 1--the "blue" granulomas. J Cutan Pathol. 2004 May;31(5):353-61. PubMed.2. Howard A, White C. Non-infectious granulomas. In: Dermatology (Bolognia J, Jorizzo J, Rapini RP, eds), 1 edn., Vol. 2. Edinburgh: Mosby, 2003: 1460-63.
3. Kovich O, Burgin S. Generalized granuloma annulare. Dermatol Online J. 2005 11(4):23. PubMed.
4. Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989 Jan;20(1):39-47. PubMed.
5. Arroyo MP. Generalized granuloma annulare. Dermatol Online J. 2003 Oct;9(4):13. PubMed.
6. Erkek E, Karaduman A, Bukulmez G, Senturk N, Ozkaya O. An unusual form of generalized granuloma annulare in a patient with insulin-dependent diabetes mellitus. Acta Derm Venereol. 2001 Jan-Feb;81(1):48-50. PubMed.
7. Sahin MT, Turel-Ermertcan A, ouml Zturkcan S, Turkdogan P. Generalized granuloma annulare in a patient with type II diabetes mellitus: successful treatment with isotretinoin. J Eur Acad Dermatol Venereol. 2006 Jan;20(1):111-4. PubMed.
8. Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996 193(4):364-8. PubMed.
9. Kakourou T, Psychou F, Voutetakis A, Xaidara A, Stefanaki K, Dacou-Voutetakis C. Low serum insulin values in children with multiple lesions of granuloma annulare: a prospective study. J Eur Acad Dermatol Venereol. 2005 Jan;19(1):30-4. PubMed.
10. Schnopp C, Tzaneva S, Mempel M, Schulmeister K, Abeck D, Tanew A. UVA1 phototherapy for disseminated granuloma annulare. Photodermatol Photoimmunol Photomed. 2005 Apr;21(2):68-71. PubMed.
11. Ozkan S, Fetil E, Izler F, Pabuccuoglu U, Yalcin N, Gunes AT. Anetoderma secondary to generalized granuloma annulare. J Am Acad Dermatol. 2000 Feb;42(2 Pt 2):335-8. PubMed.
12. Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: Part II. decreased elastic tissue. J Am Acad Dermatol. 2004 Aug;51(2):165-85; quiz 86-8. PubMed.
13. Gamo R, Ortiz-Romero P, Sopena J, Guerra A, Rodriguez-Peralto JL, Iglesias L. Anetoderma developing in juvenile xanthogranuloma. Int J Dermatol. 2005 Jun;44(6):503-6. PubMed.
14. Vaalamo M, Kariniemi AL, Shapiro SD, Saarialho-Kere U. Enhanced expression of human metalloelastase (MMP-12) in cutaneous granulomas and macrophage migration. J Invest Dermatol. 1999 Apr;112(4):499-505. PubMed.
© 2006 Dermatology Online Journal