Familial necrobiosis lipoidica not associated with diabetes
Published Web Location
https://doi.org/10.5070/D30491w5m2Main Content
Familial necrobiosis lipoidica not associated with diabetes
Elena Roche-Gamón MD, Juan J Vilata-Corell MD PhD, Manuel Velasco-Pastor MD
Dermatology Online Journal 13 (3): 26
Department of Dermatology, Hospital General Universitario, Valencia, SpainAbstract
A 42-year-old woman presented with a 12-year history of extensive yellow and erythematous plaques, round and oblong with irregular configuration and glossy atrophic central areas on the pretibial aspects of both legs. Her 45-year-old sister presented with a 7-year history of a single plaque with erythematous margins, abundant telangiectases, and an atrophic center in the lower portion of the left leg. There was no family history of type-1 or type-2 diabetes mellitus. Both patients had normal fasting glucose concentration, oral glucose tolerance test, and glucose overload test. Different treatment options including topical corticosteroids were unsuccessful. Treatment with oral fumaric acid esters was attempted but the medication was discontinued because of intolerable side effects (flushing and gastrointestinal discomfort). At present, after a follow-up of 2 years, the plaques remain unchanged. These two cases should be added to the few cases of familial nondiabetic necrobiosis lipoidica previously reported.
In 1929 Oppehheim first described necrobiosis lipoidica diabeticorum and called it dermatitis atrophicans lipoidica diabetica, but it was later renamed necrobiosis lipoidica diabeticorum by Urbach in 1932. In 1935 Goldsmith reported the first case in a nondiabetic patient. Other cases of necrobiosis lipoidica diabeticorum in nondiabetic patients were described by Meischer and Leder in 1948. Rollins and Winkelmann in 1960 also described this condition in nondiabetic patients, and a renaming of this disorder was suggested to exclude diabetes from the title [1]. Today, the term necrobiosis lipoidica (NL) is used to encompass all patients with the same clinical disease regardless of whether or not diabetes is present. However, NL is an uncommon manifestation in diabetes mellitus, occurring in about 0.3-1 percent of these patients [2]. The skin manifestation is not pathognomonic for diabetes mellitus because less than two thirds of patients with NL are diabetic. Necrobiosis lipoidica has been documented to occur prior to the onset of diabetes mellitus [3], and certainly any patient who presents with NL should be evaluated for diabetes. The condition is most common between the second and fifth decades of life, but it may be seen at any age [4]. The female-to-male ratio is approximately 3:1 [5].
Although NL has been described in nondiabetic patients [6, 7, 8], familial cases of NL seem to be extremely rare [9]. A few pairs of siblings with NL in the absence of diabetes have been previously reported [10, 11]. We here describe two female siblings with long-standing NL, not associated with diabetes mellitus, the lesions of which failed to respond to standard treatments.
Clinical synopsis
Case 1
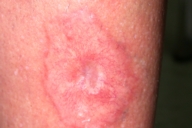 |
| Figure 1 |
|---|
| Figure 1. Necrobiosis lipoidica plaque with erythematous margins, abundant telangiectasias and an atrophic center. |
A 45-year-old woman was referred to the dermatology clinic for evaluation of a single left pretibial plaque that had been present for the previous 7 years. The plaque had been generally asymptomatic, but the patient experienced intermittent pain and occasional pruritus and burning. Her lesion failed to improve with topical corticosteroids or oral treatment with pentoxifylline. Her past medical history and family history were unremarkable. The patient specifically denied prior trauma to the affected area. Physical examination disclosed a single 4 x 3 cm plaque with erythematous margins, abundant telangiectases and an atrophic center in the pretibial area of the left leg (Fig. 1). The contour of the plaque was irregular. No erosions on the plaque were observed. A complete blood count with differential analysis, biochemical profile, fasting blood glucose level, oral glucose tolerance test, glucose overload test, and urinalysis were normal. Cardiovascular risk factors were not present.
Case 2.
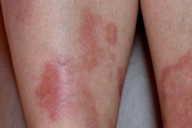
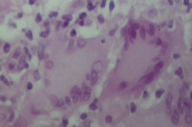
The sister of the above patient was a 42-year-old woman who was referred to us for a 12-year history of extensive plaques in the pretibial areas of both legs. The lesions persisted and worsened despite innumerable treatments, including application of high potency topical corticosteroids, intralesional injection of corticosteroids into the active margins, and oral treatment with pentoxifylline. No other sites were involved. There was no prior history of trauma. On clinical examination, bilateral pretibial plaques, with active, erythematous margins more active and atrophic centers were observed (Fig. 2). There were no similar lesions on other sites. The patient had no lymphadenopathy or hepatosplenomegaly. She was otherwise in good health, and her general examination was completely normal. Peripheral blood count, biochemical profile, fasting blood glucose level, oral glucose tolerance test, glucose overload test, and urinalysis were normal. Histopathological examination of a skin biopsy revealed a granulomatous infiltrate showing a palisaded arrangement and composed of epithelioid cells, histiocytes, and Langerhans and foreign-body-type multinucleated giant cells outlining the necrobiotic areas (Fig. 3).
In both patients, treatment with oral fumaric acid esters was started but the medication was discontinued on the second week of treatment because of intolerable side effects (flushing and gastrointestinal discomfort). Since then, other treatment options were not attempted. At present, after a follow-up of 2 years, lesions have remained unchanged.
Discussion
The etiology of NL remains unknown and although the disease classically has been associated with type 1 diabetes mellitus and less frequently with type 2, it is also observed in nondiabetic adults [8]. In patients with diabetes mellitus, there is some controversy regarding the effect of glucose control on the clinical course of the plaques that seem to occur and persist independent of the degree of control of hyperglycemia [12]. Recently, NL has been postulated as a marker of cardiovascular risk [13] or as a guiding sign for the detection of patients with fasting glucose intolerance.
Two histological subtypes of NL have been defined: a granulomatous form, which is the most frequent presentation and is found in association with diabetes, and an infrequent, necrobiotic variant that predominantly manifests in nondiabetic patients. Our second patient presented the granulomatous form of the disease; 12 years of follow-up has revealed no anomalies in glucose metabolism.
Because the pathophysiology of necrobiosis is not understood, it is difficult to design rational therapy. To date, no proven effective therapy for NL has been implemented. The standard treatment is topical or intralesional administration of corticosteroids, but numerous agents have been reported for NL, with varying degrees of success. First-line therapy includes application of potent topical corticosteroids and the injection of corticosteroids into the active borders of the established plaques. Other therapies used in case series include topical retinoids [14], nicotinamide, pentoxifylline [15], aspirin and dipyridamole, perilesional heparin, phototherapy [16], topical growth factors and prostaglandins, mycophenolate mofetil, cyclosporin [17], stanozolol, clofazimine, hyperbaric oxygen, fumaric acid esters [18], topical tacrolimus [19], infliximab [20], and pulsed dye laser therapy.
The present report of a new case of familial NL in nondiabetic patients might raise the possibility of a genetic basis for the development of this cutaneous lesion.
Acknowledgment: We thank Dr Marta Pulido for editing the manuscript and editorial assistance.
References
1. Rollins TG, Winkelmann RK. Necrobiosis lipoidica granulomatosis. Necrobiosis lipoidica diabeticorum in the nondiabetic. Arch Dermatol 1960;82:537-543.2. Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. A clinical and pathological investigation of 171 cases. Arch Dermatol 1966;93:272-281.
3. Ellenberg M. Diabetic complications without manifest diabetes. JAMA 1963;183:926-930.
4. Verrotti A, Chiarelli F, Amerio P, Morgese G. Necrobiosis lipoidica diabeticorum in children and adolescents: a clue for underlying renal and retinal disease. Pediatr Dermatol 1995;12:220-223.
5. Lowitt MH, Dover JS. Necrobiosis lipoidica. J Am Acad Dermatol 1991;25:735-748.
6. Csaszar A, Daroczy J, Szenasi P, Anda L, Toth L, Hosszufalusi N, Karadi I, Kalabay L, Romics L. Necrobiosis lipoidica without diabetes mellitus (diagnostic and therapeutic possibilities). [Article in Hungarian]. Orv Hetil 1989;130:2141-2145.
7. Wee SA, Possick P. Necrobiosis lipoidica. Dermatol Online J 2004;10:18.
8. O'Toole EA, Kennedy U, Nolan JJ, Young MM, Rogers S, Barnes L. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol 1999;140:283-286.
9. Seviour PW, Elkeles RS. Necrobiosis lipoidica in two diabetic sisters. Clin Exp Dermatol 1985;10:159-161.
10. Findlay GH, Morrison JG, de Beer HA. Non-diabetic necrobiosis lipoidica. Hitherto unrecognized papulonecrotic, nodulo-ulcerative and familial forms of the disease. S Afr Med J 1981;59:323-326.
11. Ho KK, O'Loughlin S, Powell FC. Familial non-diabetic necrobiosis lipoidica. Australas J Dermatol 1992;33:31-34.
12. Cohen O, Yaniv R, Karasik A, Trau H. Necrobiosis lipoidica and dibetic control revisited. Med Hypotheses 1996;46:348-350.
13. Leal Hernandez M, Abellan Aleman J, Vicente Martinez R, Martinez Crespo J. Lipoid necrobiosis in an asymptomatic patient: marker of cardiovascular risk? [Article in Spanish]. Aten Primaria 2004;33:474-475.
14. Boyd AS. Tretinoin treatment of necrobiosis lipoidica diabeticorum. Diabetes Care 1999;22:1753-1754.
15. Noz KC, Korstanje MJ, Vermeer BJ. Ulcerating necrobiosis lipoidica effectively treated with pentoxifylline. Clin Exp Dermatol 1993;18:78-79.
16. De Rie MA, Sommer A, Hoekzema R, Neumann HA. Treatment of necrobiosis lipoidica with topical psoralen plus ultraviolet A. Br J Dermatol 2002;147:743-747.
17. Stanway A, Rademaker M, Newman P. Healing of severe ulcerative necrobiosis lipoidica with cyclosporin. Australas J Dermatol 2004;45:119-122.
18. Kreuter A, Knierim C, Stucker M, Pawlak F, Rotterdam S, Altmeyer P, Gambichler T. Fumaric acid esters in necrobiosis lipoidica: results of a prospective noncontrolled study. Br J Dermatol 2005;153:802-807.
19. Harth W, Linse R. Topical tacrolimus in granuloma annulare and necrobiosis lipoidica. Br J Dermatol 2004;150:792-794.
20. Kolde G, Muche JM, Schulze P, Fischer P, Lichey J. Infliximab: a promising new treatment option for ulcerated necrobiosis lipoidica. Dermatology 2003;206;180-181.
© 2007 Dermatology Online Journal