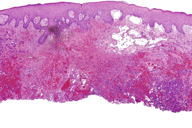
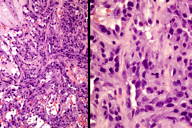
Figures 2 and 3. Histologic sections
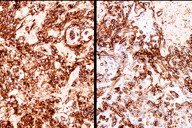
Figure 4. Immunohistochemical staining (A) CD31 and (B) D2-40
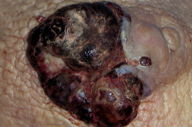
A 54-year-old woman presented a peri-areolar nodule located in the skin of the right breast. Clinical examination showed a 6 x 5 cm exophytic, lobed, ulcerated, and bleeding nodule (Figure 1). The patient reported that the tumor had grown gradually over a period of 3 months. The patient had been diagnosed 8 years prior to presentation with infiltrating ductal carcinoma of the right breast (pT2NO). This tumor was treated with partial mastectomy (conservative surgery) and lymph node dissection, then subsequently received 30 tangent field radiotherapy sessions to the breast for a total dose of 45 Gy. The rest of her cutaneous exam was normal. There was no family history of any similar tumor. Histological sections are show in Figures 2, 3, and 4.
 |
 |
| Figure 1 | Figure 2 |
|---|---|
| Figure 1. Showed a lesion exophytic, lobed, ulcerated and bleeding at the touch Figures 2 and 3. Histologic sections Figure 4. Immunohistochemical staining (A) CD31 and (B) D2-40 |
|
 |
 |
| Figure 3 | Figure 4 |
|---|
The histological sections showed an ulcerated skin tumor, occupying the entire dermis and infiltrating breast structures (Figure 2). The neoplasm was composed of vascular channels containing erythrocytes (Figure 3A). These vascular channels were formed by cells with large, irregular, hyperchromatic nuclei. Also seen were solid areas composed of spindle cells with atypia, with abundant mitotic activity (Figure 3B). Immunohistochemical study showed positivity in tumor cells for CD31, CD34, D2-40, and for high proliferative activity (Ki67 positive in 70% of tumor cells) (Figure 4A and 4B). The diagnosis was made of secondary angiosarcoma of the breast Grade III. Although the surrounding skin clinically has the appearance of lymphedema, we were unable to find evidence of this histologically in the areas of skin between the tumor and normal skin.
The tumor was treated with radical mastectomy without any evidence of recurrence after seven months of follow-up.
Angiosarcoma of the breast was first described in 1887 by Schmidt. It is a rare neoplasm corresponding to 0.05 percent of primary tumors of the breast and about 8 percent of sarcomas of the breast [1]. There are two types of angiosarcomas, primary and secondary. The primary angiosarcoma of the breast originates in the breast parenchyma affecting the skin in advanced stages. It usually affects women aged 20-40 years [2, 3]. The secondary angiosarcoma of the breast originates in the skin, then infiltrates the breast parenchyma in patients at older ages than patients with primary angiosarcoma of the breast. These patients have usually been treated with radiotherapy or have acquired chronic lymphedema after mastectomy with axillary dissection (Stewart-Treves syndrome) [4]. The post-radiotherapy angiosarcomas of the breast may occur in patients treated with mastectomy and radiotherapy and affect the chest wall. In patients treated with conservative surgery and radiation, it preferentially affects the skin and breast parenchymal involvement is very rare. In these cases occurring after conservative surgery and radiotherapy, the angiosarcomas are multifocal and correspond to histological Grade II-III.
Studies show that patients undergoing partial mastectomy (conservative surgery) with axillary dissection and radiotherapy are 7 times more at risk of developing a secondary angiosarcoma than patients who have total mastectomy with axillary dissection and radiotherapy. This increased risk may be related to subclinical lymphedema caused by disruption of lymphatic structures of the breast parenchyma when performing partial surgery; this could increase the effect of radiotherapy on the development of angiosarcoma [5].
The latency period between surgery, radiation, and the appearance of angiosarcoma has been reported between 2 and 30 years [6, 7, 8]. In our case the latent period was 8 years.
Angiosarcomas of the breast are classified into three grades: Grade I (well differentiated) forms vascular channels containing red blood cells lined by endothelial cells with large hyperchromatic nuclei, Grade II (moderately differentiated) has 25 percent solid tumor areas, Grade III (poorly diferentiated) has more than 50 percent solid tumor areas with spindle cells, foci of necrosis, and intense mitotic activity. Histologic grade influences the prognosis for primary angiosarcoma. However, grade does not always influence the outcome when the tumor is a secondary angiosarcoma [4].
The differential diagnosis of angiosarcoma includes hemangioma, Phyllode tumor, stromal sarcoma, metaplastic carcinoma, myoepithelioma, and fibrosarcoma [9].
The treatment is mastectomy without axillary dissection because this tumor has a low incidence of axillary mestastasis [10]. Treatment with chemotherapy and radiotherapy is not clear, although adjuvant radiotherapy can reduce local recurrence [11].
In our patient underwent a mastectomy with extended margins, these margins remain free of tumor 7 months after surgery.
© 2011 Dermatology Online Journal