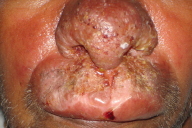
A 40-year-old man presented with nodules and plaques of the nose and upper lip, progessive over 1½ years.
 |
| Figure 1 |
|---|
| Figure 1. Diffuse indurated swelling involving nose and upper lip |
A 40-year-old male presented with a diffuse erythematous swelling involving the upper lip and lower part of the nose that was of 1½ years duration. Initially the patient had complaints of nasal stuffiness and blockage, followed by the appearance of a small nodule on the nose. This gradually increased in size and spread to involve the upper lip. The lesion was asymptomatic and the patient's only concern was cosmetic disfigurement. On clinical examination, there was a diffuse erythematous indurated swelling of the upper lip and lower part of the nose. On the surface of the lesion crusting was present along with sero-sanginous discharge (Fig. 1). The surrounding areas of bilateral cheeks and the upper part of the nose showed erythema. Oral mucosa and palate were normal. There was no lymphadenopathy.
 |
 |
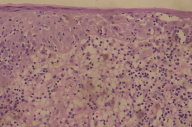
| Figure 2 | Figure 3 |
|---|---|
| Figure 2. Photomicrograph showing lymphocytic infiltration of epidermis and dermis Figure 3. Immunohistochemistry showing predominant CD3+ cells. |
|
 |
| Figure 4 |
|---|
| Figure 4. Immunohistochemistry showing sparse CD20+ cells |
All the routine biochemical and hematological investigations were within normal limits. X-ray and CT-scan of the region, and ultrasonography of abdomen were normal. Biopsy from the upper lip showed lymphocytic infiltration in the epidermis along with a dense cellular infiltrate in the dermis. The infiltrate was composed of small lymphocytes with eosinophilic to clear cytoplasm, irregular nuclei, and occasional mitotic figures (Fig. 2). A few plasma cells and polymorphs were present. Immunohistochemistry showed predominant infiltration of CD3+ cells (Fig. 3) and a few CD20+ cells (Fig. 4).
Cutaneous T cell lymphoma (CTCL), generally classified as a type of non-Hodgkin lymphoma, represents a spectrum of diseases composed of malignant clonal helper T lymphocytes of the CD4 phenotype. The incidence of CTCL is 0.3 per 105 person-years [1]. CTCL is approximately twice as common in men as in women, while blacks have twice the incidence of whites. Most cases are diagnosed in the fifth and sixth decades.
Three classical cutaneous phases of CTCL patches, infiltrated plaques, and tumors were described by Bazin in 1876 [2]. The disease may progress through each of these phases, that frequently overlap or occur simultaneously. In 5 percent to 10 percent of cases, the tumor phase may be the initial disease presentation without evolution, originally termed the "d'emblee" variant by Videl and Brocq in 1885 [3].
Tumor stage CTCL may arise from patches, from plaques, or de novo. Lesions are typically violaceous, exophytic, mushroom-shaped tumors that preferentially affect the face and body folds (Fig. 3) [4]. Lesions often undergo ulceration or necrosis and secondary infection. Pruritus may decrease in intensity during this stage. Over 50 percent of deaths from CTCL are caused by Staphylococcus aureus or Pseudomonas aeruginosa sepsis.
The diagnosis of CTCL is usually made by recognizing the characteristic clinical manifestations of the disease plus routine histology. In difficult cases, a preliminary diagnosis may be supported by additional laboratory tests such as immunophenotyping, flow cytometry, and T cell receptor (TCR) gene rearrangement analysis. Light microscopy of hematoxylin and eosin-stained sections from involved skin is still the diagnostic gold standard, but the diagnosis in early stages may be difficult [4].
Clinical evaluation of CTCL patients includes a complete history and physical examination, emphasizing the types of skin lesions, body surface area, lymph node, liver, and spleen involvement. Baseline tests should include a complete blood cell count, peripheral blood flow cytometry for T cell subsets, serum chemistries (liver and renal function tests, calcium, phosphorus, uric acid, and lactate dehydrogenase), chest radiography, and biopsy or fine-needle aspiration of palpable lymph nodes. Additional staging procedures for patients with advanced disease include computed tomography scan of the abdomen and pelvis, gallium scan, and bone marrow biopsy.
A number of staging systems for CTCL have been proposed. The simplest and most widely used system, adopted by Lamberg et al. incorporates the tumor-node-metastasis (TNM) system [5]. This staging system combines both clinical and histopathologic perspectives. The most important clinical predictive factors for survival include patient age, T classification, and the presence of extracutaneous disease [6]. The long-term survival of patients with stage IA (T1, N0) is similar to the expected survival of a race-, age-, and sex-matched control population [7]. Relative risk for death is 2.2 in stage IB/IIA disease, 3.9 in stage IIB/III disease, and 12.8 in stage IV disease [6]. The present patient belonged to stage IIB, that is, tumor stage without nodal or systemic metastasis [8].
The differential diagnosis in this case could be Wegner granulomatosis and midline granuloma. These could be ruled out due to the clinical course, CD3+ positivity in majority of lymphocytic infiltrates [9], and histopathological findings.
Treatment regimens in CTCL include skin-directed therapies such as psoralen with UVA irradiation (PUVA), topical chemotherapy with mechlorethamine (nitrogen mustard) and carmustine (BCNU), and electron beam radiation, as well as systemic therapies such as chemotherapy, photopheresis, and interferons. A stage-adapted approach to CTCL therapy is used most often. Radiation therapy is the initial treatment of choice for patients with localized tumor-stage CTCL [8]. Subsequent management depends on response to radiotherapy.
© 2008 Dermatology Online Journal