An unusual presentation of primary cutaneous nocardiosis at a rare site: Succesful treatment with a modified Welsh regimen
Published Web Location
https://doi.org/10.5070/D30zs6j6tbMain Content
An unusual presentation of primary cutaneous nocardiosis at a rare site: Succesful treatment with a modified Welsh regimen
Praveen Kumar S MD DNB, T K Sumathy MD MNAMS, A L Shyam Prasad MD, Gayathri Devi D R MD, K N Shivaswamy MD DNB, C Ranganathan
MD DVD
Dermatology Online Journal 17 (12): 1
MS Ramaiah Medical College and Teaching Hospital, Bangalore, Kamataka, IndiaAbstract
INTRODUCTION: Primary cutaneous nocardiosis can present in various forms. Clinically, it can present as acute infection (abscess or cellulitis), mycetoma, or sporotrichoid infection. Mycetoma over the back is rare. CASE REPORT: We herein describe a case of primary cutaneous nocardiosis presenting as a mycetoma, caused by Nocardia brasiliensis. The patient had extensive lesions over the back, which can be attributed to the fact that the patient, being an agriculturist, has been exposed to recurrent trauma while carrying firewood and soiled sacks. He responded well to a modified Welsh regimen. Initially, within 2 cycles, the patient showed dramatic improvement clinically, wherein the sinuses, granulation tissue, and induration were no longer apparent. However, the patient showed a small discharging sinus at the end of 3rd pulse, so a total of 6 cycles were given. An additional 2 months of maintenance phase treatment with cotrimoxazole and rifampicin were given. On follow-up, the patient showed no recurrence at 6 months. CONCLUSION: We report a case of primary cutaneous nocardiosis presenting as a mycetoma on the back. Enlisting the help of a microbiologist allowed us to isolate the causative organism. Early recognition and prompt treatment prevents unwarranted surgical debridement and complications.
Introduction
Mycetoma is a chronic granulomatous disease that affects the skin, subcutaneous tissue, fascia, muscle, and occasionally the underlying bone and adjacent organs [1]. Historically, mycetoma was recognized as a disease entity in 1842 by Dr. John Gill, while working in Madurai, India. For this reason, the term “Madura Foot” originated. Pinoy, in 1913, subdivided the mycetomas into two groups: those caused by bacteria of the actinomycetes group and those caused by the eumycetes or “true fungi.” Mycetoma is characterized by the triad of tumefaction, discharging sinuses, and the presence of grains formed by the colonies of causal organisms [2]. Cutaneous Nocardiosis can present as a mycetoma. This mode of presentation is commonly reported from India. The other clinical variants are acute superficial skin and soft tissue infections and a lymphocutaneous infection. The first description of Nocardia came from a French veterinarian, Edmond Nocard, in 1888 in relation to bovine farcy [3]. The genus Nocardia belongs to the order Actinomycetales, a group of aerobic, gram-positive filamentous bacteria. The organism is geophillic and is found in soil and decaying plant parts. Isolation of the organisms is a tedious process and requires the expertise of a microbiologist. A high degree of clinical suspicion and repeated examination of exudates for Nocardia granules are both important for making an early diagnosis. An early diagnosis, particularly for nocardial mycetoma cases, is very desirable because invasive surgical measures, such as deep tissue debridement or amputation, are unnecessary if chemotherapy is used early. Herein, we report a case of mycetoma caused by Nocardia brasilensis, presenting at an unusual site, over the back, which showed a dramatic response to a modified Welsh regime.
Case report
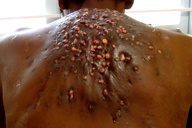 | 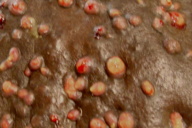 |
| Figure 1 | Figure 2 |
|---|---|
| Figure 1. Indurated plaque over back with multiple sinuses and granulation tissue Figure 2. Close-up view of lesions | |
A 30-year-old male farmer presented with a 4-year history of multiple painful, draining sinuses and nodules on the back. A history of trauma with a wooden splinter of a mat was elicited. The lesion initially started as a small pustule that progressed over 4 years to involve the whole of his back. On examination, a hyperpigmented indurated plaque studded with multiple granulomatous nodules, discharging sinuses, and scars were seen over the upper back (Figures 1 and 2). Local lymph nodes were inconspicuous. A KOH preparation from the discharge did not show any fungal elements. A gram stain of the pus revealed long, beaded gram-positive branching filaments.
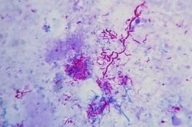 |  |
| Figure 3 | Figure 4 |
|---|---|
| Figure 3. Kinyoun staining shows thin long branching weakly acid-fast bacillus. Figure 4. Culture on Lowenstein-Jensen media shows dry, wrinkled, granular colonies. | |
Modified Kinyoun stain revealed weakly acid-fast bacilli (Figure 3). Initial cultures from the discharge on McConkey agar and SDA agar showed no growth. A repeat biopsy of deeper tissue, cultured on Lowenstein-Jensen medium, revealed dry, granular, wrinkled orange color colonies after 4 weeks (Figure 4). The isolate was identified as Nocardia brasiliensis by its ability to hydrolyze casein, esculin, hypoxanthine, and tyrosine. Catalase, urease, and citrate reduction tests were positive. The isolate fermented glucose but not arabinose.
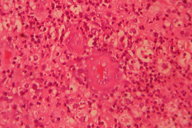 |  |
| Figure 5 | Figure 6 |
|---|---|
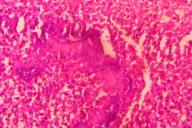
| Figure 5. Dermis shows inflammatory infiltrate with giant cells. Figure 6. Grains seen amidst inflammatory infiltrate with splendor-hoeppli phenomenon. | |
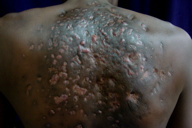
Histopathological examination revealed multiple microabscesses with actinomycotic colonies, around which splendor-hoeppli material was evident (Figures 5 and 6). X-ray of the chest revealed no bony abnormalities. Baseline blood count, urinalysis, renal/liver function tests, and pure tone audiometry were within normal limits. The patient was started on a modified Welsh regime. Six cycles of simultaneous administration of amikacin (15 mg/kg/day) divided into two daily doses for 3 weeks and cotrimoxazole (7 and 35 mg/kg/day) continuously for 5 weeks were administered. A gap of 2 weeks was given after each cycle of amikacin. The patient was monitored regularly with blood counts, renal function tests, liver function tests, and pure tone audiometry. Within 2 cycles the induration, granulation tissue, discharge, and sinuses were not clinically apparent (Figure 7).
 |  |
| Figure 7 | Figure 8 |
|---|---|
| Figure 7. Lesions after 2nd cycle showed complete healing of sinuses with hyperpigmented scarring. Figure 8. Small sinus with granulation tissue after 3rd cycle | |
 |  |
| Figure 9 | Figure 10 |
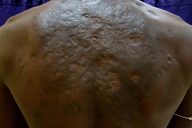
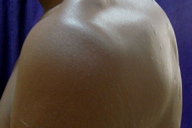
|---|---|
| Figure 9. Lesions after 6 cycles showed hyper pigmented scarring Figure 10. Lesions at 6 months of follow-up showed a complete healing of lesions with hyperpigmented scarring | |
However, at the end of the 3rd pulse patient developed a small discharging sinus over the back. At this time hemoglobin was 8.4 gm% and his pure tone audiometry showed a dip at 8 khz, high frequency sloping hearing loss. ENT consultation was requested and their opinion was that the hearing loss was reversible. We decided, therefore, to give a total of 6 pulses. At the end of the 6th pulse, our patient had no new lesions and on examination only hyperpigmented scarring was seen over the back (Figures 8 and 9). An additional maintenance phase of 2 months of cotrimoxazole and rifampicin were given. On follow-up, no new lesions were seen after 6 months. Biopsy at 6 months of follow-up showed no evidence of mycetoma (Figure 10).
Discussion
Nocardial infections can occur worldwide, particularly in tropical/subtropical environments. In India, the first report of N. brasiliensis infection appeared in 1964. The incidence of Nocardia mycetoma in India varies from 5.2 percent to 35 percent of mycetomas [4]. Among the several species of Nocardia causing cutaneous infections, Nocardia brasiliensis is the most common species isolated. The infective process is initiated by local trauma, such as a puncture wound caused by a thorn or a splinter during a variety of outdoor activities. The saprophytic Nocardia species, present on these materials, are implanted in the puncture wound and initiate the infection. The abscess enlarges by direct extension of the nocardial filaments through tissues. Dissemination from primary cutaneous nocardiosis to distant anatomic sites is rare. In our patient, extensive lesions involving the back were noted and initiation was related to a pricking injury by the splinter of a wooden mat. The extensive involvement can also be attributed to the fact that the patient would have sustained repeated trauma while carrying firewood/soiled sacks on the shoulders. The patient referred to the lesion as an ant hill on his back. The first written reference to mycetoma is contained in the Indian religious book Atharva Veda, in which it is described as “Padavalmika.” Padavalmika means foot-anthill, which refers to the report by patients that pain associated with mycetoma is reminiscent of ant hill activity.
Mycetomas are implantation infections caused by organisms that have been introduced directly into the dermis or subcutaneous tissue through a penetrating injury. This holds true for both bacterial and deep mycotic infections. Mycetoma is characterized by the presence of tumefaction, draining sinuses, and sclerotia, which are vegetative aggregates of the etiologic agent. Sclerotia are also called granules or grains. An infection caused by non-classical agents might be considered mycetome-like or “pseudomycetoma.” In pseudomycetoma, the clinicopathologic process is similar to mycetoma, but sclerotia are absent in either the discharge or tissue. “Paramycetoma” are similar to pseudomycetoma but here fungi are absent. “Minimycetoma” refers to the presence of clinically demonstrable tumors, microsinuses, and micrograins. Primary cutaneous nocardiosis can present as either 1) a superficial acute skin and soft tissue infection with abscess or cellulitis, 2) a lymphocutaneous (sporotrichoid) [5] infection, 3) a deeper infection (mycetoma), and 4) a disseminated infection with skin involvement. The genus Nocardia comprises several species of clinical importance. N. brasiliensis is the main pathogenic organism for primary cutaneous infection, followed by N. asteroides, which causes fulminant systemic infections. Other pathogenic species include N. otitidis-caviarum, N. transvalensis, N. farcinia and N. nova. The usual sites of involvement are the hands and feet. Other sites of occurrence like the scalp, shoulder, and upper back, which correspond to the usual sites of carrying loads by agricultural workers, have also been reported. Unlike eumycetoma, these lesions are acute in onset, more inflammatory, and associated with tenderness. The sinuses are usually surrounded by a raised border or are punched out. The granules are less than 1mm in size and yellowish-white. Nocardia brasiliensis has a propensity to involve the underlying bone and osteolytic lesions are seen radiologically. Nocardia brasiliensis is known for dissemination, through the hematogenous route. Tenderness and inflammation differentiate this from fungal infections. Nocardia asteroides, in particular, may cause a localized abscess without dissemination. Nocardia is an opportunistic pathogen and is often found in people with progressive chronic disease or with a defect in T-lymphocyte mediated immune responses, including AIDS patients and solid organ transplant recipients. It has also been reported with Cushing syndrome, diabetes, and corticosteroid use.
Diagnoses
In cutaneous Nocardiosis the organisms are most commonly isolated from deeper tissues on repeated examinations. Grams staining and modified Kinyoun staining are the techniques for the identification of Nocardia. Direct smears typically show Gram-positive, beaded, branching filaments that are usually acid-fast [6]. In general the colonies are chalky white or have a cottonball appearance because of the presence of abundant aerial filaments. Nocardia species will grow on most non-selective media used routinely for the culture of bacteria, fungi, and mycobacteria. In mixed infections, when the pus is taken from superficial tissues, the proliferation of rapidly growing bacteria obscures the growth of Nocardia. Selective media such as Thayer-Martin agar with antibiotics increase the yield. Growth may take 48 hours to several weeks, but typical colonies are seen after 3-5 days. Once the organism is isolated various tests can differentiate the different species. Nocardia brasiliensis hydrolyses casein and tyrosine but not xanthine [7]. Nocardia otitidis/caviarum hydrolyses only xanthine, whereas Nocardia asteroids hydrolyses casein, tyrosine and xanthine.
Treatment
Several antibiotics have been used in the treatment of actinomycetoma. Cotrimoxazole, dapsone, streptomycin, sulfadoxine-pyrimethamine, rifampicin, and amoxicillin-clavulanic acid have all been found effective. Some combinations that have been found useful are cotrimoxazole and streptomycin, cotrimoxazole and amikacin, cotrimoxazole and dapsone, cotrimoxazole and penicillin, dapsone and ampicillin, tetracycline or chloramphenicol, dapsone and amikacin and dapsone and streptomycin [8]. Surgery may be required in patients unresponsive to medical therapy alone. The possible synergistic effects of the antimicrobials form the basis of these combination therapies. The efficacy of the antimicrobial synergistic activity can be confirmed by in vitro studies. A combination of an aminoglycoside and cotrimoxazole has been used with good results. Mahgoub treated 81 patients with streptomycin and cotrimoxazole for 4-24 months; 66 patients were cured or greatly improved. With success, physicians have used various combinations of these drugs to treat actinomycetomas. Moreover, the use of multidrug therapy is preferable in order to avoid drug resistance and eradicate residual infection. Ramam et al [9] used a 2-step regimen consisting of an intensive phase with cotrimoxazole, penicillin, and gentamycin for 5 to 7 weeks, followed by maintenance therapy with amoxycillin and cotrimoxazole. Rapid response was seen in all 7 patients treated with this regimen and 6 patients who completed therapy healed completely. Oral amoxicillin-clavulanate 875/125 mg administered every 12 hours was used successfully by Bonifaz et al [8] to treat 21 cases of actinomycetoma caused by Nocardia species. Sharma et al [10] have reported a single case of actinomycetoma of the foot who had a complete treatment failure with the sulfomethoxazole-trimethoprime-dapsone combination. However, the patient was successfully treated with an amikacin and dapsone combination, without any side effects.
Welsh Regime [1, 11]
A cycle of Welsh regime consists of simultaneous administration of amikacin (15 mg/kg/day) divided into two daily doses for 3 weeks and cotrimoxazole (trimethoprime/sulfomethoxazole) 7 and 35 mg/kg/day, respectively, continuously for 5 weeks. A gap of 2 weeks is given for amikacin in each cycle after 3 weeks of administration.
Modified Welsh regime [12]
Oral rifampicin (10 mg/kg/day) is added as a third drug along with trimethoprime and sulfomethoxazole (T-S), in the welsh regime. Rifampicin is said to be a good option in the treatment of actinomycetoma cases resistant to first line treatment. Rifampicin is reported as a good second line drug for actinomycetoma. Furthermore, rifampicin is an important component in the treatment of tuberculosis and leprosy. In the setting of a tropical country like India, medical personnel are familiar with its safety, tolerability, and efficacy in a large number of patients. Pulikot et al used a combination of rifampicin, cotrimoxazole, and dapsone to treat actinomycetoma of the sole in a 10-year-old male patient with a good outcome. In our patient we used the modified welsh regimen to treat the patient. Our patient was regularly monitored with a blood count, renal function tests/liver function tests, and pure tone audiometry before each cycle. The hematological side effects of cotrimoxazole were followed. Problems may occur because of either trimethoprime or sulfamethoxazole. Trimethoprim-induced cytopenias are more frequent, dose-dependent, usually mild, and easily reversed, whereas those related to sulfamethoxazole are rare, idiosyncratic, and may be severe. Regular monitoring of the blood count is helpful in early identification of this adverse effect. The duration of treatment required to cure actinomycetoma is not clearly defined. Prolonged treatment is recommended to prevent relapses. In our patient, 6 cycles of modified Welsh regimen were given, since the patient had a recurrence with a small sinus after the 3rd pulse. After 6 cycles of the modified Welsh regimen, there was no recurrence of lesions and the patient had clinical and functional improvement. There were no sinuses or discharge. Our patient was given an additional 2 months of maintenance treatment with cotrimoxazole and rifampicin. There was no recurrence on follow-up after 4 months. In our patient, he had to travel a long distance for treatment in our hospital. Therefore, we coordinated his care with a local doctor from a primary health center (PHC) near his home. The injections of amikacin were continued by the doctor in the PHC after his discharge. This method reduced the duration of hospital stay for the treatment.
Conclusion
Cutaneous nocardiosis can present in various forms. A high degree of clinical suspicion and the expertise of a microbiologist is required to diagnose and isolate the causative organism. Early treatment of mycetoma prevents complications and the need for extensive debridement. Combination therapies are better than monotherapy with cotrimoxazole, both to clear and to prevent recurrence. We herein report this patient who had extensive lesions on the back caused by Nocardia brasiliensis. He showed a dramatic response to treatment with a modified Welsh regimen. Successful medical treatment of mycetoma usually necessitates 4 to 24 months of therapy. A drastic reduction in the sinuses, granulation tissue, induration, discharge, and swelling were noted within 2 months of therapy and complete cure was achieved in 6 months. Our case also highlights the logistics and feasibility of treatment on an outpatient basis.
ACKNOWLEDGEMENT: The authors would like to thank Dr. Peerapur (Microbiology Department, BLDE Medical College, Bijapur) for his contributions in isolating Nocardia brasiliensis.
References
1. Welsh, M.D. Mycetoma: current concepts in treatment. International Journal of Dermatology. June 1991;30(6):387-390. [PubMed]2. Somanath P, Shantveet G U, Megha S U, Umabala P, et al. Mycetoma in south India: retrospective analysis of 13 cases and description of two cases caused by unusual pathogens: Neoscytalidium dimidatum and Aspergillus flavus. The International Society of Dermatology. 2010;49:1289-1296. [PubMed]
3. Inamadar AC, Palit A. Primary cutaneous nocardiosis: A case study and review. Indian J Dermatol Venerol Leprol. December 2003; 69(6):386-391. [PubMed]
4. Talwar P, Sehgal SC. Mycetomas in North India. Sabouraudia 1979;17:287-91. [PubMed]
5. Sharma NL, Mahajan VK, Agarwal S, Katoch VM, Das R, Kashyap M, Gupta P, Verma GK. Nocardial mycetoma; Diverse clinical presentations. Indian J Dermatol Venerol Leprol. Nov-dec;74(6): 635-640. [PubMed]
6. Marcelo E.C., Maria F., Villafane F. Nocardiosis: a review. Int J infect Dis. 2003;7:243-250. [PubMed]
7. Kiska DL, Hicks K, Pettit DJ. Identification of medically relevant Nocardia species with a abbreviated battery of tests. Journal of Clinical microbiology. April 2002;40(4):1346-1351. [PubMed]
8. Chavez G, Estrada R, Bonifaz A. Perianal actinomycetomas experience of 20 cases. Int J Dermatol 2002;41:491-3. [PubMed]
9. Ramam M, Radhakrishna B, Taru G, Vinod K S. A modified two-step treatment for actinomycetoma. Indian J Dermatol Venereol Leprol. 2007; 73(4): 235-239 [PubMed]
10. Sharma N, Mendiratta V, Sharma RC, at al. Pulse therapy with amikacin and dapsone for the treatment of actinomycotic foot - a case report. J Dermatol. 2003;30:742-747. [PubMed]
11. Kaliswaran A.V., Senthamilselvi G., Janaki C., Janaki V.R. Therapeutic response in mycetoma - A study of different regimes. Indian J Dermatol. 2003;48(3): 154-59.
12.Mahajan P.M., Pradhan S.N., Belgaumkar V.A., Gosavi A.P. et al. Modified Welsh regimen: a promising therapy for actinomycetoma. Journal of drugs in dermatology. Sept 2008;7(9):853-6. [PubMed]
© 2011 Dermatology Online Journal

