Acanthosis nigricans in the setting of niacin therapy
Published Web Location
https://doi.org/10.5070/D34gd131pkMain Content
Acanthosis nigricans in the setting of niacin therapy
Rachael Hartman MD, Taylor DeFelice MD MPH, Julia Tzu MD, Shane Meehan MD, Miguel Sanchez MD
Dermatology Online Journal 17 (10): 11
Department of Dermatology, New York University, New York, New York Abstract
We report the case of a 63-year-old obese man with a rapid-onset of widespread acanthosis nigricans (AN) in the setting of having recently initiated treatment with niacin for dyslipidemia. Although obesity and insulin-resistance are risk factors for AN, AN associated with endocrine dysfunction tends to have a more gradual onset and limited involvement. Owing to our patient’s age, the rapid onset, and extensive distribution of his eruption, we initially were concerned about paraneoplastic AN. However, an evaluation for a malignant condition was negative. The timing of the onset of our patient’s eruption within several months of starting niacin therapy is consistent with niacin-induced AN. Niacin is known to cause rapidly progressive, widespread AN that is reversible upon discontinuation of the medication. We discuss the pathogenesis of AN, which is thought to be the final common manifestation of stimulation of different subtypes of tyrosine kinase receptors by various epidermal growth factors.
History
A 63-year-old man presented to the dermatology clinic at Bellevue Hospital Center in July, 2009, with darkening of the skin of his neck, groin, axillae, chest, back, hands, and feet. The lesions were asymptomatic and developed abruptly two months prior to his initial clinic visit. He denied fevers, chills, night sweats, weight loss, and weight gain during the past year.
The patient has coronary artery disease, which in 2005 resulted in a myocardial infarct that may have been exacerbated by cocaine use, hypertension, and dyslipidemia. Other medical conditions include paroxysmal atrial fibrillation, vertigo/syncope, anemia, diverticulosis, benign prostatic hypertrophy, gastroesophageal reflux, and osteoarthritis. The patient started taking niacin 500 mg twice daily in October, 2008, for the treatment of dyslipidemia, but he discontinued it after one month because of flushing. In March, 2009, niacin was started again at the same dose, but because of a recurrence of flushing, was changed four months later to an extended release formulation (Niaspan 1000 mg daily). At the time of initial presentation, he also was being treated with hydrochlorothiazide, lisinopril, aspirin, atorvastatin, metoprolol, tamsulosin, esomeprazole, omega-3 fatty acids, coenzyme Q10, and a multivitamin.
A punch biopsy was obtained from a plaque on the chest. The skin lesions have been treated with triamcinolone ointment and hydrocortisone cream and the discoloration has slightly faded.
Physical examination
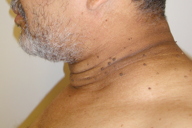 | 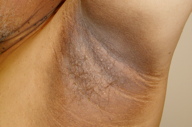 |
| Figure 1 | Figure 2 |
|---|
The patient is an obese man with a body mass index of 31.2 kg/m². Velvety, hypertrophic, tan-to-brown plaques are present on the neck, both axillae, the inner thighs, lateral aspect of the chest, lower back, antecubital fossae, and dorsal aspects of the hands and feet. The palms, soles, and mucous membranes are not involved. There also were numerous acrochordons on the neck and in the axillae.
Laboratory data
The hemoglobin was 12.0 g/dL, hematocrit 36.9 percent, and peripheral eosinophilia 5.7 percent. Basic metabolic and hepatic function panels were normal. Hemoglobin A1c was normal, but a fasting insulin level was elevated at 21 µIU/mL (normal <17 µIU/mL). The high-density lipoprotein was 26 mg/dL. Levels of thyroid stimulating hormone, lactate dehydrogenase, serum prostate specific antigen, and carcinoembryonic antigen were normal. A chest radiograph did not show abnormal findings. Colonoscopy demonstrated diverticulosis and upper endoscopy showed mild gastritis and fundic gland polyps.
Histopathology
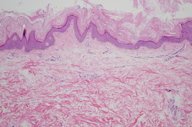 |
| Figure 3 |
|---|
There is papillomatosis with basal layer hyperpigmentation and basketweave orthokeratosis, which is slightly thickened. There is also a sparse, superficial, perivascular lymphocytic infiltrate.
Discussion
Acanthosis nigricans is characterized by symmetric, velvety-to-verrucous, hyperkeratotic, hyperpigmented plaques that can develop on any part of the body, but most commonly involve the neck and flexural areas [1]. The disorder is disproportionately observed in darkly-pigmented populations. The highest prevalence has been found in Native Americans (34.2%), followed by African Americans (13.3%), Latinos (5.5%), and Caucasians (less than 1%) [1, 2, 3].
Acanthosis nigricans can be inherited or acquired as a manifestation of an endocrinopathy, malignant condition, or medication. It is most commonly diagnosed in patients with insulin-resistance that results from obesity, type 2 diabetes mellitus, and polycystic ovary syndrome [1, 4]. Acanthosis nigricans caused by endocrine dysfunction tends to have a gradual onset and relatively mild cutaneous changes [1]. In one study, AN was diagnosed in 74 percent of obese patients. Higher fasting plasma insulin levels were found in patients with AN than were found in obese persons without acanthotic changes. These findings suggest that the presence of AN is a valuable cutaneous marker of hyperinsulinemia and insulin resistance in obesity [5] and underscores the importance of screening obese persons with AN for occult diabetes mellitus, glycosylated hemoglobin levels, and plasma insulin levels to detect insulin resistance.
Paraneoplastic AN is much rarer than is the benign form. In comparison to other types of AN, the skin lesions of paraneoplastic AN erupt more abruptly, progress more rapidly, are more widespread, are more likely to be pruritic, and more often involve the oral mucosa. The skin changes may be accompanied by other cutaneous stigmata of malignant conditions, especially tripe palms and the sign of Leser-Trélat. Adenocarcinoma of the gastrointestinal tract, particularly gastric cancer, is the most common of the many malignant conditions in which paraneoplastic AN has been reported. In one study, AN was diagnosed simultaneously with the associated malignant condition in 61 percent of cases and predated the diagnosis of malignant conditions in 17 percent of the cases [1]. A search for malignant conditions is therefore warranted in adults with sudden-onset of AN.
Acanthosis nigricans can also be caused by therapeutic agents, especially niacin, insulin, glucocorticoids, estrogen, and protease inhibitors [1, 6-9]. Several reports describe patients who developed rapidly progressive, widespread AN without an underlying malignant condition within four to seven months of starting niacin therapy [6, 7, 8, 9]. A man was reported with AN caused by nicotinic acid who had normal fasting glucose and insulin levels but a moderate degree of glucose-stimulated hyperinsulinemia [8]. In these cases, the lesions gradually regressed after discontinuation of niacin and, in some cases, recurred when nicotinic acid was readministered [6, 7, 8, 9]. It has been hypothesized that niacin causes AN by inducing insulin resistance and/or by interfering with epidermal cholesterol synthesis, which, in turn, leads to abnormal barrier function, increased DNA synthesis, and epidermal hyperplasia [8].
Stimulation of epidermal keratinocytes and dermal fibroblasts by growth factors is considered to be important in the pathogenesis of AN. In benign AN, the stimulating factor is probably insulin or insulin-like growth factor (IGF). At low concentrations, insulin binds to classic insulin receptors. It regulates carbohydrate, lipid, and protein metabolism and only weakly stimulates growth. However, insulin resistance leads to compensatory hyperinsulinemia. At high concentrations, insulin binds to IGF-1 receptors on fibroblasts and keratinocytes. These receptors are similar in size and structure to insulin receptors but more strongly promote proliferation of skin cells. Notably, hyperinsulinemia also may increase the levels of free IGF-1 in the circulation [10, 11]. The growth factor associated with paraneoplastic AN is believed to be transforming growth-factor (TGF)-α, which acts via the epidermal growth factor receptor (EGFR). High levels of both TGF-α and EGFR may be present in gastric adenocarcinomas. AN lesions associated with gastric cancers resolve or improve after reduction of previously elevated TGF-α levels following tumor resection. Furthermore, EGFR expression reportedly is increased in tissue from paraneoplastic AN [10, 11].
Acanthosis nigricans also has been linked to a variety of syndromes, most of which are associated with either insulin resistance (e.g., leprechaunism, Rabson-Mendenhall, Berardinelli-Seip, Dunnigan, Alstrom, and HAIR-AN syndromes) or fibroblast growth factor (FGF) defects (e.g., Beare-Stevenson syndrome, Crouzon syndrome with AN, thanatophoric dysplasia, and severe achondroplasia with developmental delay and AN) [11]. Activation of FGF receptors (FGFR) may also be the mechanism by which palifermin, a recombinant FGF receptor ligand that is used to decrease chemotherapy-related mucositis, produces transient but dramatic AN-like changes [12]. Because IGFR, EGFR, and FGFR are all tyrosine kinase receptors, AN may be the manifestation of several processes that share a common pathway [10, 11]. The predilection of AN for skin folds suggests that perspiration and/or friction contributes to the growth of acanthotic lesions [10].
Histopathologic examination of AN shows hyperkeratosis and epidermal papilomatosis. The name AN is a misnomer since there usually is minimal or no acanthosis. Pseudohorn cysts may be present. The hyperpigmentation observed in AN is related to hyperkeratosis rather than increased melanocytes or melanin deposition. There is no appreciable inflammatory infiltrate in the dermis [1, 10].
Treatment should be directed at the underlying cause. Endocrinopathy-associated AN improves with weight reduction and the treatment of insulin resistance [1]. Dietary fish oils containing omega-3 fatty acids may be beneficial in cases of generalized AN and lipodystrophic diabetes [10, 13]. Antidiabetic drugs that reduce insulin, such as rosiglitazone and octreotide, as well as those that do not, such as metformin, have shown mixed results in the treatment of lesions in obese patients [10, 14-17]. Paraneoplastic AN usually regresses after successful eradication of the underlying malignant condition but may recur with relapse or metastasis of the cancer [1]. Niacin-induced AN resolves with withdrawal of the medication or substitution of an analog, such as acipimox [6, 7, 8, 9]. Both topical and systemic retinoids have been used to effectively treat AN [10, 17-20]. Keratolytics, such as ammonium lactate, can be valuable as adjunctive therapy [10, 18]. Resolution of lesions has also been accomplished with repeated treatments with the long-pulsed alexandrite laser [10, 21].
References
1. Schwartz RA. Acanthosis nigricans. J Am Acad Dermatol 1994; 31:1 [PubMed]2. Stoddart ML, et al. Association of acanthosis nigricans with hyperinsulinemia compared with other selected risk factors for type 2 diabetes in Cherokee Indians: the Cherokee Diabetes Study. Diabetes Care 2002; 25:1009 [PubMed]
3. Stuart CA, et al. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989; 87:269 [PubMed]
4. Kahn CR, et al. The syndromes of insulin resistance and acanthosis nigricans: insulin-receptor disorders in man. N Engl J Med 1976; 294:739 [PubMed]
5. Hud JA, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol 1992; 128:941 [PubMed]
6. Coates P, et al. Resolution of nicotinic acid-induced acanthosis nigricans by substitution of an analogue (acipimox) in a patient with type V hyperlipidemia. Br J Dermatol 1992; 126:412 [PubMed]
7. Elgart ML. Acanthosis nigricans and nicotinic acid. J Am Acad Dermatol 1981; 5:709 [PubMed]
8. Stals H, et al. Acanthosis nigricans caused by nicotinic acid: case report and review of the literature. Dermatology 1994; 189:203 [PubMed]
9. Tromovitch TA, et al. Acanthosis nigricans-like lesions from nicotinic acid. Arch Dermatol 1964; 89:222 [PubMed]
10. Higgins SP, et al. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J 2008; 14:2 [PubMed]
11. Torley D, et al. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002; 147:1096 [PubMed]
12. Lane SW. et al. Palifermin-induced acanthosis nigricans. Intern Med J 2007; 37:417 [PubMed]
13. Sheretz EF. Improved acanthosis nigricans with lipodystrophic diabetes during dietary fish oil supplementation. Arch Dermatol 1988; 124:1094 [PubMed]
14. Bellot-Rojas P, et al. Comparison of metformin versus rosiglitazone in patients with acanthosis nigricans: a pilot study. J Drugs Dermatol 2006; 5:884 [PubMed]
15. Romo A, Benavides S. Treatment options in insulin resistance obesity-related acanthosis nigricans. Ann Pharmacother 2008;42:1090 [PubMed]
16. Tankova T, et al. Therapeutic approach in insulin resistance with acanthosis nigricans. Int J Clin Pract 2002;56:578 [PubMed]
17. Walling HW, et al. Improvement of acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol 2003; 2:677 [PubMed]
18. Blobstein SH. Topical therapy with tretinoin and ammonium lactate for acanthosis nigricans associated with obesity. Cutis 2003; 71:33 [PubMed]
19. Darmstadt GL, et al. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol 1991; 127:1139 [PubMed]
20. Katz RA. Treatment of acanthosis nigricans with oral isotretinoin. Arch Dermatol 1980; 116:110 [PubMed]
21. Rosenbach A, et al. Treatment of acanthosis nigricans of the axillae using a long-pulsed (5-msec) alexandrite laser. Derm Surg 2004; 30:1158 [PubMed]
© 2011 Dermatology Online Journal

