Malignant melanoma in African-Americans
Published Web Location
https://doi.org/10.5070/D33k77p755Main Content
Malignant melanoma in African-Americans
Filamer D Kabigting1, Fern P Nelson MD1, C Lisa Kauffman MD2, Geanina Popoveniuc MD3, Constantin A Dasanu MD PhD4, Doru T Alexandrescu MD1,2
Dermatology Online Journal 15 (2): 3
1. Dermatology Clinical Trials Unit, University of California at San Diego, San Diego, California. mddoru@hotmail.com2. Georgetown Dermatology, Washington, DC
3. Department of Medicine, Washington Hospital Center, Washington, DC
4. Department of Hematology/Oncology, St. Francis Medical Center, Hartford, Connecticut
Abstract
Although relatively uncommon, malignant melanoma in African-Americans and other minority ethnic populations represents an aggressive disease highly associated with invasive lesions and a more advanced stage of disease at diagnosis, and consequently with a decreased survival compared with Caucasians. Data on biology of melanoma in African-Americans is very limited, which complicates the analysis of epidemiological information, as well as identification of accurate prognostic variables. This review article explores critical features of melanoma in African-Americans that distinguish it from disease seen in Caucasians, including the clinical presentation, histological patterns, prognostic indicators, and etiology. Emerging data from biologic and genetic studies will also be discussed, raising the possibility that melanoma in pigmented skin may represent molecular distinct cancers that are inherently more aggressive. Improved understanding of the unique manifestations of melanoma in African-Americans, and its underlying tumor biology, will help improve clinical detection, optimize preventative measures through public health education, and potentially lead to the development of novel targeted therapeutic approaches.
Background
Melanoma is the sixth most common cancer in the United States and the single most common one among young adults 25-29 years old [1]. Recent trends demonstrate that incidence rates have increased 2.4 percent per year in the last decade [2]. Lifetime risk of developing melanoma in whites is currently estimated at 1 in 50, compared to 1 in 1000 in African-Americans. Although darker-pigmented populations are consistently reported to have lower risk for melanoma [3], possibly related to protection from ultraviolet radiation (UVR) provided by melanin [4], these groups are consistently shown to have a worse outcome than Caucasians. Between 1996 and 2004, the 5-year survival rates for melanoma reported from the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) Program ranged from a high of 93.5 percent among white women to a low of 71.2 percent among black men [2].
Differences in melanoma survival have been attributed to multiple factors, including inadequate patient education, limited access to medical resources, lack of suspicion by providers for melanoma in skin of color patients, presence of unique genetic alterations, and occurrence of lesions in unconventional locations. This anatomic distribution is different from the one classically seen in whites, and is oftentimes inconsistent with the "ABCDE" rules exhibited by typical melanomas [5]. Definitive explanations for this aggressive course with an inherent tendency to metastasize, however, are lacking. Limited data exists on the pathogenesis and progression melanoma in ethnic minority populations, partially due to relatively lower incidence of disease in these groups. Much of the existing literature and previous public health interventions have focused predominantly on Caucasians, although increasing efforts are now being made to characterize melanoma in ethnic minorities. This interest resulted from accumulating data which show a worse outcomes and a higher mortality in patient groups like African-Americans, who are significantly more likely to present at an advanced stage. Continued research to understand the unique manifestations of melanoma in African-Americans holds significant promise to improve clinical detection, develop targeted therapies, and optimize preventative measures, so that, ultimately, differences in the overall survival can be corrected across ethnic populations developing melanoma.
Clinical presentation
Demographic features
Minority ethnic groups exhibit a lower incidence of melanoma than Caucasians, with rates per 100,000 persons of 1.00 in African-Americans, 1.5 in Asian/Pacific Islanders, 2.9 in American Indians/Alaska Natives and 4.05 in Hispanics [6]. The average annual age-adjusted incidence of melanoma according to the California Cancer Registry is highest in Whites (Males 17.2/100,000 and Females 11.3/100,000), followed by Hispanics (2.8 and 3.0), Asians (0.9 and 0.8), and African Americans (1.0 and 0.7, respectively) [7].
Some studies report males to have a slightly higher incidence of malignant melanoma than females, regardless of ethnicity, with the exception of Hispanics for whom incidence is similar between genders [6, 7, 8]. For example, data extracted from SEER records indicated major differences in age-adjusted incidence between men and women among non-Hispanic whites (31.4 per 100,000 males and 20.6 per 100,000 females) and American Indians/Alaska Natives (4.1 per 100,000 males and 2 per 100,000 females), while African-Americans and Asians exhibited quite a narrow range of variance (1.1 per 100,000 males and 0.9 per 100,000 females in African-Americans) [7]. Other studies suggest the ratio female/male patients being higher than 1 in all minority ethnic groups [5, 9, 10, 11].
In terms of age, median ages at diagnosis range from 50 to 65 in darker-skinned ethnic groups [5, 7, 9-14], with an observed tendency to the right end of the interval in African Americans [5, 9, 10, 12], while median ages in Hispanics were skewed to the left [7, 9, 10].
Anatomical distribution of lesions
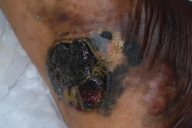
The frequency distribution of melanoma by anatomic site differs between Caucasians and ethnic minority populations. While Caucasians have a predilection to develop lesions on sun-exposed surfaces, including face and neck, blacks have lesions predominantly located on sun-protected mucosal and acral sites [5, 7, 11-12, 15-16], particularly the foot, which is speculated to be related to traumatic injury (Fig. 1) [17, 18]. This pattern of distribution has also been observed in Puerto Rican [7, 9] and Asian series [7, 20, 21]. The incidence of primary tumors in acral sites among African-Americans is estimated between 60 percent [14, 15, 21] to 73 percent [22]. This figure is similar to rates for plantar melanoma reported in black South Africans (73%) [23], black Ugandans (65%) [24], as well as Japanese (25-35%) [20] and Chinese (46%) [21] series. Despite this predilection for melanoma in acral sites, the incidence of acral melanoma in African-Americans and Caucasians has been shown to be similar [25, 26], a fact obscured by the lower overall incidence of melanoma in blacks, especially in non-acral locations. In fact, ALM is considered to be the only subtype of melanoma found with equal frequency across all races [26, 27].
Stage at diagnosis
African-Americans and other ethnic minorities with melanoma are more likely to present at an advanced disease stage compared to Caucasians [8, 15, 18, 33-36]. These findings persist even when adjusting for the histologic type and site of primary lesion [8]. Byrd et al. [12] reported in their series of 649 patients from the Washington Hospital Center that African-Americans were three times more likely to have stage III/IV disease at presentation compared to whites (32.1% vs. 12.1%, respectively). This also corresponded with thicker lesions, with mean Breslow depths of 2.75 mm in African-Americans vs. 1.16 mm in whites. Other data by Metzger et al. reveal that African-Americans are three times more likely to have distant metastases present at diagnosis compared to Caucasians (14% vs. 4%, respectively) [36].
Histopathologic features
Acral lentiginous melanoma (ALM) is the major subtype of melanoma observed among African-Americans, Hispanic, and Asian populations [5, 7, 11, 14, 16, 28], with estimates that it accounts for greater than 50 percent of all melanomas among both African-Americans [7, 16, 25, 29] and Black South Africans [14], compared to roughly 5 percent in Caucasians, who predominantly have superficial spreading melanoma (SSM) [10]. Histologically, ALM is characterized by a proliferation of atypical melanocytes along adnexal structures and the dermoepidermal junction (DEJ) in sites like the palms and soles where eccrine sweat ducts are richly concentrated [30, 31].
 |
| Figure 2 |
|---|
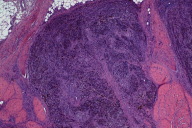
| Figure 2. Melanoma on the plantar surface of a patient with pigmented skin shows an extensive lesion with a 4 cm diameter, presence of ulceration, and deep subcutaneous invasion. |
Compared to other melanoma subtypes, ALM is more likely to be detected at advanced tumor size and stage [32, 33, 34] (Fig. 2). This is attributed to high rates of misdiagnosis that delay appropriate management. In fact, an estimated one-third to one-half of all cases of ALM are incorrectly diagnosed at initial presentation as the more commonly appearing, benign skin lesions including warts, dermatophyte infections, ulcers, callus, traumatic wounds, keratoacanthoma, and hematomas [35-37]. High rates of misdiagnoses are especially associated with unpigmented lesions and lesions involving the nail [36]. Such diagnostic delays prolong the critical period between evaluation and treatment, leading ultimately to higher incidence of thicker, more invasive tumors at presentation [36].
Histopathologic markers associated with a delayed diagnosis or the presence of advanced disease, including a large tumor depth and ulceration, are more commonly seen in African-Americans compared to Caucasians. One study by Crowley et al. reported that greater than 40 percent of primary tumors in African-Americans had evidence of ulceration, nearly double the rate seen in whites [38]. In another series of African-Americans from Charity Hospital New Orleans (CHNO), Bellows et al. found histological evidence of ulceration in nearly 60 percent of primary tumors in African-Americans. Of 16 ulcerative lesions identified, 13 were associated with stage III/IV disease (P<0.05), and ulceration was found in a multivariate analysis to predict an unfavorable prognosis independently of other factors and after adjusting for disease stage [5].
Median tumor depth at presentation has also been shown to be approximately twice as large in African-Americans compared to whites (1.2 mm vs. 0.66 mm) [9]. This is consistent with patterns observed by Byrd et al, where the mean Breslow depth was significantly greater in African-Americans vs. Caucasians (2.75 mm and 1.16 mm, respectively) [12]. Studies from South Africa report even more impressive data, where mean Breslow depth exceeding 6-7 mm were found in black South Africans, nearly doubled the figure seen in whites [14,23].
Prognostic factors
 |
| Figure 3 |
|---|
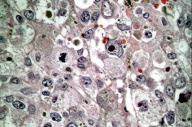
| Figure 3. Histological features of acral melanoma occurring in pigmented skin include the presence of prominent mitoses, large vacuolated nuclei. (HE, x400) |
As early as 1975, Rippey et al. conducted one of the earliest studies examining the relationship between histological features of primary melanoma lesions and outcome. They reported that blacks had a greater tendency to present with invasive lesions, but that, generally, a similar set of prognostic indicators related to outcome in whites was also relevant to African-Americans. This includes size and shape of the primary lesion, extent of invasion, presence of ulceration, and mitotic activity (Fig. 3) [39].
Subsequent studies largely corroborated these findings. The most common clinico-pathologic variables thought to influence prognosis in patients with melanoma include age, location, tumor thickness, presence of ulceration, stage of disease, and race [5, 13, 38, 40]. Additionally, the Clark level (P<0.01), lack of pigment production (P<0.01), vascular invasion (P<0.01), presence of microscopic satellites (P<0.01), and high mitotic rate (P<0.01) have also been reported as predictors of survival [30]. Whether or not race is a significant independent prognostic variable remains controversial; some studies report it to be predictive of survival, along with Clark level and Breslow depth [41], whereas others note its lack of statistical significance upon controlling for tumor thickness [42].
Whether or not ALM, as a subtype in itself, confers a worse prognosis remains highly debatable. There is some data to suggest that ALM is not an independent risk factor for prognosis after adjustment for race and histologic stage [26, 33, 40, 43-47]. Ridgeway et al. reported in their series of 56 patients with ALM that melanoma subtype was not an independent prognostic indicator. Here, patients with ALM, superficial spreading melanoma (SSM), and nodular melanoma (NM) had similar disease free survival (DFS) and overall survival (OS) upon correction for tumor thickness [26]. Some authors suggest that the delayed diagnosis commonly associated with ALM accounts for a worse survival, rather than an inherently more aggressive nature of the tumor itself [44].
Considerations of race as an independent prognostic factor in survival remain controversial with competing evidence reported. The most compelling evidence is derived from several studies that show a worse prognosis in African-Americans with melanoma, even after controlling for key variables like stage of disease, tumor thickness, ulceration, anatomic site, and histological type [9, 15, 40]. A statistically significant higher mortality in African-Americans persists even after adjustment for socioeconomic status [10]. This data suggests that perhaps melanoma in African-Americans manifests in a more aggressive course.
Data refuting the role of race as an independent prognostic factor was also generated [5]. Hemmings et al. [13] could not demonstrate any survival differences in non-white vs. white patients, after adjusting for the stage at diagnosis. This finding is compatible with the data of O'Leary et al., who evaluated 93 patients diagnosed with subungual melanoma, among whom 12 percent were African-American [48]. By utilizing a multivariate analysis of the group of patients presenting with stage I disease, race was not determined to be able to predict patient survival. Instead, the Clark and Wihm's level (p=0.035), clinical stage (p=0.045), and ulceration (p=0.03) were deemed to be reliable prognostic variables. Therefore, a suggestion can be made that the clinical stage is a more important prognostic factor than the race. Increased efforts to improve early detection of melanoma in dark skin patients are therefore warranted, and may contribute to a shift of stage toward earlier lesions, associated with a more favorable prognosis.
Etiology
While ultraviolet radiation (UVR) exposure is frequently incriminated as a causative factor for melanoma in Caucasians, its role is more controversial in darkly-pigmented populations like African-Americans who have a significant predilection to develop melanoma on sun-protected locations, such as the plantar, palmar, subungual and mucosal surfaces. In a series of 126 patients with histologically-proven ALM, Phan et al. reported no evidence of overexposure to UVR in patients examined, nor any predisposing genetic traits [22]. Additional evidence for the existence of other causative factors aside from UV radiation is a non-linear relationship between melanoma and skin pigmentation reported by Halder et al. [4].
Other reported risk factors for melanoma in blacks include albinism, burn scars, immunosuppresion, radiation therapy, and trauma [15]. The latter is often considered a risk factor for ALM given the observation that lesions occur on weight bearing areas. While previous reports found a preceding history of trauma related to later development of ALM in 13 percent of cases [22], others suggest that these patients likely had unrelated, preexisting foot conditions prior to injury that the traumatic event later drew attention to [49].
The role of preexisting pigmented lesions remains unclear, especially in the context of melanomas arising in acral locations where direct sun exposure is highly limited to facilitate malignant transformation. Nagore et al. reported in their series of 46 patients with ALM that only 8.3 percent of cases had histopathological evidence of pre-existing nevi [50]. On the other hand, the association between melanocytic nevi (MN) and melanomas occurring on non-acral sites is better characterized. In their series of 131 patients with invasive melanoma predominantly on the trunk, Massi et al. reported that an associated MN was found in approximately 20 percent of cases [51].
The lower incidence of this skin cancer in African-Americans, Asians, and Hispanics may be the result of a protective effect exerted by darker cutaneous pigmentation [7, 52]. Increased melanin density considerably reduces the frequency of melanoma and other sun-induced skin cancers [34], possibly due to the fact that an increased pigmentation is negatively correlated with UV radiation-induced DNA damage. Although an inverse correlation exists between the melanin concentration of the skin and the level of DNA damage produced by UV exposure, the melanin content per se does not seem to be associated with an increased efficiency of the DNA removal mechanisms [53]. Therefore, the absolute amount and distribution of melanin are critical constitutive factors in determining photoprotection by blocking the UV penetrance into the skin and the resultant carcinogenesis [7].
Given that melanoma in African-Americans is associated with different risk factors and presents with distinct clinical features, it should come as no surprise that there is increasing data to suggest that unique biologic and genetic determinants of melanoma may exist between ethnic groups. Diverse molecular pathways are responsible for melanoma, and the genetic alterations involved in oncogenesis vary by anatomic site and levels of sun exposure [54]. While mutations in BRAF and NRAS genes are frequently found in tumors associated with pre-existing nevi as well as melanomas of intermittently sun-exposed skin [54], they are less common in melanomas of mucosal and acral sites, which are proportionately higher in African-Americans [55, 56, 57].
Furthermore, melanomas occurring on acral skin, specifically the ALM subtype, show lower levels of p16 expression compared to other melanoma types [58]. This decrease in p16 expression is associated with poorer survival (P=0.05), which may suggest enhanced aggressiveness of associated subtypes like ALM. Vuhahula et al. [59] reported consistent findings in his series of patients with acral melanoma, noting that half of cases had loss of p16 staining. Furthermore, other features associated with increased tumor aggressiveness include a high proliferative activity (increased Ki-67 expression), low p53 staining positivity, and high microvessel density. Among white patients with melanoma, these markers are known negative prognostic factors that indicate tumor progression, although data for blacks is more limited [60, 61]. Other genetic aberrations with a differential expression depending on factors such as race, primary tumor location, and melanoma subtype include amplifications and mutations of the KIT gene [62], c-myc, p53 [63], and downstream components of the BRAF-RAS pathway including cyclin-dependent kinase 4(CDK4), and cyclin D1 (CCND1) [54]. This data reflects the genetic heterogeneity found in melanoma, revealing that subtle biological differences may account for a wide spectrum of disease manifestation that is observed clinically in individual populations.
Conclusions
Although Caucasians have a sixteen-fold greater incidence of melanoma, African-Americans with the disease are more likely to present at advanced stages and suffer worse outcomes. Current data suggests that survival differences between ethnic groups are complex, and not simply due to solitary factors like unfavorable histology, melanoma stage at diagnosis, or depth of primary tumor at presentation. While persistent differences exist in the melanoma burden, recent trends demonstrate an improved survival in African-Americans, providing encouragement. Critical to these gains has been the role of public health education. By increasing awareness and recognition of melanoma in pigmented skin, earlier detection as well as minimized rates of misdiagnoses can be achieved. Continued efforts, however, are still needed to further narrow disparities in melanoma survival. Educational programs directed to minority communities, as well as physicians, should encourage the performance of meticulous and thorough physical examinations for all patients, with special emphasis on examining acral and mucosal regions in African-American, Hispanic, and Asian patients.
The oncogenesis of melanoma in African-Americans remains unclear. It appears, however, that well-documented risk factors relevant to melanoma development in whites, namely sun exposure, are less critical in African-Americans. There is a good reason to believe that melanoma is a genetically heterogenous disease, with diverse etiologies.
Further biologic and genetic studies are required to elucidate the particular behavior of melanoma among different racial/ethnic groups. This may explain why some groups, like African-Americans, appear to have a more aggressive course and poorer survival than others. As these genetic pathways to melanoma development continue to unravel, the potential to develop targeted molecular therapies for this disease also becomes a closer reality.
References
1. Cancer Epidemiology in Older Adolescents & Young Adults. SEER AYA Monograph Pages 53-57.2007.2. Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK (eds). SEER Cancer Statistics Review, 1975-2005, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site, 2008.
3. American Cancer Society. 2008 Cancer Facts and Figures.
4. Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995 Jan 15;75(2 Suppl):667-73. [PubMed]
5. Bellows CF, Belafsky P, Fortgang IS, Beech DJ. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-6. [PubMed]
6. [SEER, Table 2] SEER Cancer Statistics Review 1975-2004; Section 16 Table 2. Melanoma of the skin (invasive) age adjusted SEER incidence rates by year, race and sex. Available at: http:/seer.cancer.gov/publications. Accessed February 25, 2008
7. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data, 1988-1993. Cancer Causes Control. 1997;8:246-52. [PubMed]
8. Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-60; quiz 761-4. Review. [PubMed]
9. Cormier JN, Xing Y, Ding M, Lee JE, Mansfield PF, Gershenwald JE, Ross MI, Du XL. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-14. [PubMed]
10. Zell JA, Cinar P, Mobasher M, Ziogas A, Meyskens FL Jr, Anton-Culver H. Survival for patients with invasive cutaneous melanoma among ethnic groups: the effects of socioeconomic status and treatment. J Clin Oncol. 2008;26:66-75. [PubMed]
11. Swan MC, Hudson DA. Malignant melanoma in South Africans of mixed ancestry: a retrospective analysis. Melanoma Res. 2003;13:415-9. [PubMed]
12. Byrd KM, Wilson DC, Hoyler SS, Peck GL. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-4; discussion 142-3. [PubMed]
13. Hemmings DE, Johnson DS, Tominaga GT, Wong JH. Cutaneous melanoma in a multiethnic population: is this a different disease? Arch Surg. 2004;139:968-72; discussion 972-73. [PubMed]
14. Hudson DA, Krige JE. Melanoma in black South Africans. J Am Coll Surg. 1995;180:65-71. [PubMed]
15. Reintgen DS, McCarty KS Jr, Cox E, Seigler HF. Malignant melanoma in the American black. Curr Surg. 1983;40:215-7. [PubMed]
16. Giraud RM, Rippey E, Rippey JJ. Malignant melanoma of the skin in Black Africans. S Afr Med J. 1975;49:665-8. [PubMed]
17. Camain R, Tuyns AJ, Sarrat H, Quenum C, Faye I. Cutaneous cancer in Dakar. J Natl Cancer Inst. 1972;48:33-49. [PubMed]
18. Davies JNP, Tank R, Meyer R, Thurston P. (1968). Cancer of the integumentary tissues in Ugandan Africans. J Natl Cancer Inst. 1968;4:31-51. [PubMed]
19. Pantoja E, Llobet RE, Roswit B. Melanomas of the lower extremity among native Puerto Ricans. Cancer. 1976;38:1420-3. [PubMed]
20. Ishihara K, Saida T, Yamamoto A; Japanese Skin Cancer Society Prognosis and Statistical Investigation Committee. Updated statistical data for malignant melanoma in Japan. Int J Clin Oncol. 2001;6:109-16. [PubMed]
21. Collins RJ. Melanoma in the Chinese of Hong Kong. Emphasis on volar and subungual sites. Cancer. 1984;54:1482-8. [PubMed]
22. Phan A, Touzet S, Dalle S, Ronger-Savlé S, Balme B, Thomas L. Acral lentiginous melanoma: a clinicoprognostic study of 126 cases. Br J Dermatol. 2007;157:311-8. [PubMed]
23. Hudson DA, Krige JE, Stubbings H. Plantar melanoma: results of treatment in three population groups. Surgery. 1998;124:877-82. [PubMed]
24. Lewis MG. Malignant melanoma in Uganda. The relationship between pigmentation and malignant melanoma on the soles of the feet). Br J Cancer. 1967;21:483-95. [PubMed]
25. Stevens NG, Liff JM, Weiss NS. Plantar melanoma: is the incidence of melanoma of the sole of the foot really higher in blacks than whites? Int J Cancer. 1990;45:691-3. [PubMed]
26. Ridgeway CA, Hieken TJ, Ronan SG, Kim DK, Das Gupta TK. Acral lentiginous melanoma. Arch Surg. 1995;130:88-92. [PubMed]
27. Barnhill RL, Mihm MC Jr. The histopathology of cutaneous malignant melanoma. Semin Diagn Pathol. 1993;10:47-75. [PubMed]
28. Elder DE. Skin cancer. Melanoma and other specific nonmelanoma skin cancers. Cancer. 1995;75:245-56. [PubMed]
29. Vázquez M, Ramos FA, Sánchez JL. Melanomas of volar and subungual skin in Puerto Ricans. A clinicopathologic study. J Am Acad Dermatol. 198410:39-45. [PubMed]
30. Phan A, Touzet S, Dalle S, Ronger-Savlé S, Balme B, Thomas L. Acral lentiginous melanoma: histopathological prognostic features of 121 cases. Br J Dermatol. 2007;157:311-8. [PubMed]
31. Reed R, editor. Acral lentiginous melanoma. New York: John Wiley & Sons, Inc., 1976.
32. Coleman WP 3rd, Loria PR, Reed RJ, Krementz ET. Acral lentiginous melanoma. Arch Dermatol. 1980;116:773-6. [PubMed]
33. Kuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in caucasians: clinical features, histopathology and prognosis in 112 patients. Br J Dermatol. 2000;143:275-80. [PubMed]
34. Dwyer PK, Mackie RM, Watt DC, Aitchison TC. Plantar malignant melanoma in a white Caucasian population. Br J Dermatol. 1993;128:115-20. [PubMed]
35. Bristow IR, Acland K. Acral lentiginous melanoma of the foot and ankle: A case series and review of the literature. J Foot Ankle Res. 2008;1:11. [PubMed]
36. Metzger S, Ellwanger U, Stroebel W, Schiebel U, Rassner G, Fierlbeck G. Extent and consequences of physician delay in the diagnosis of acral melanoma. Melanoma Res. 1998;8:181-6. [PubMed]
37. Soon SL, Solomon AR Jr, Papadopoulos D, Murray DR, McAlpine B, Washington CV. Acral lentiginous melanoma mimicking benign disease: the Emory experience. J Am Acad Dermatol. 2003;48:183-8. [PubMed]
38. Crowley NJ, Dodge R, Vollmer RT, Seigler HF. Malignant melanoma in black Americans. A trend toward improved survival. Arch Surg. 1991;126:1359-64; discussion 1365. [PubMed]
39. Rippey JJ, Rippey E, Giraud RM. Pathology of malignant melanoma of the skin in Black Africans. S Afr Med J. 1975;49:789-92. [PubMed]
40. Slingluff CL Jr, Vollmer R, Seigler HF. Acral melanoma: a review of 185 patients with identification of prognostic variables. J Surg Oncol. 1990;45:91-8. [PubMed]
41. Søndergaard K, Olsen G. Malignant melanoma of the foot. A clinicopathological study of 125 primary cutaneous malignant melanomas. Acta Pathol Microbiol Scand [A]. 1980;88:275-83. [PubMed]
42. McGovern VJ. The nature of melanoma. A critical review. Cancer. 1983;52:1748-53. [PubMed]
43. Weyers W, Euler M, Diaz-Cascajo C, Schill WB, Bonczkowitz M. Classification of cutaneous malignant melanoma: a reassessment of histopathologic criteria for the distinction of different types. Cancer. 1999;86:288-99. [PubMed]
44. Cascinelli N, Zurrida S, Galimberti V, Bartoli C, Bufalino R, Del Prato I, Mascheroni L, Testori A, Clemente C. Acral lentiginous melanoma. A histological type without prognostic significance. J Dermatol Surg Oncol. 1994;20:817-22. [PubMed]
45. Urist MM, Balch CM, Soong S, Shaw HM, Milton GW, Maddox WA. The influence of surgical margins and prognostic factors predicting the risk of local recurrence in 3445 patients with primary cutaneous melanoma. Cancer. 1985;55:1398-402. [PubMed]
46. Barnes BC, Seigler HF, Saxby TS, Kocher MS, Harrelson JM. Melanoma of the foot. J Bone Joint Surg Am. 1994;76:892-8. [PubMed]
47. Fletcher JR, White CR Jr, Fletcher WS. Improved survival rates of patients with acral lentiginous melanoma treated with hyperthermic isolation perfusion, wide excision, and regional lymphadenectomy. Am J Surg. 1986;151:593-8. [PubMed]
48. O'Leary JA, Berend KR, Johnson JL, Levin LS, Seigler HF. Subungual melanoma. A review of 93 cases with identification of prognostic variables. Clin Orthop Relat Res. 2000;378:206-12. [PubMed]
49. Briggs JC. The role of trauma in the aetiology of malignant melanoma: a review article.Br J Plast Surg 1984, 37:514-516. [PubMed]
50. Nagore E, Pereda C, Botella-Estrada R, Requena C, Guillén C. Acral lentiginous melanoma presents distinct clinical profile with high cancer susceptibility. Cancer Causes Control. 2009;20:115-9. Epub 2008 Aug 29. [PubMed]
51. Massi D, Carli P, Franchi A, Santucci M. Naevus-associated melanomas: cause or chance? Melanoma Res. 1999;9:85-91. [PubMed]
52. Kato T, Kumasaka N, Suetake T, Tabata N, Tagami H. Clinicopathological study of acral melanoma in situ in 44 Japanese patients. Dermatology. 1996;193:192-7. [PubMed]
53. Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003;17:1177-9. [PubMed]
54. Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-47. [PubMed]
55. Akslen LA, Puntervoll H, Bachmann IM, Straume O, Vuhahula E, Kumar R, Molven A. Mutation analysis of the EGFR-NRAS-BRAF pathway in melanomas from black Africans and other subgroups of cutaneous melanoma. Melanoma Res. 2008;18:29-35. [PubMed]
56. Lang J, Mackie RM. Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes. J Invest Dermatol. 2005;125:575-9. [PubMed]
57. Saldanha G, Potter L, DaForno P, Pringle JH. Cutaneous Melanoma Subtypes Show Different BRAF and NRAS Mutation Frequencies. Clin Cancer Res. 2006;12:4499-505. [PubMed]
58. Chana JS, Grover R, Wilson GD, Hudson DA, Forders M, Sanders R, Grobbelaar AO. An analysis of p16 tumour suppressor gene expression in acral lentiginous melanoma. Br J Plast Surg. 2000;53:46-50. [PubMed]
59. Vuhahula E, Straume O, Akslen LA. Frequent loss of p16 protein expression and high proliferative activity (Ki-67) in malignant melanoma from black Africans. Anticancer Res. 2000;20:4857-62. [PubMed]
60. Straume O, Sviland L, Akslen LA. Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clin Cancer Res. 2000;6:1845-53. [PubMed]
61. Pavey SJ, Cummings MC, Whiteman DC, Castellano M, Walsh MD, Gabrielli BG, Green A, Hayward NK. Loss of p16 expression is associated with histological features of melanoma invasion. Melanoma Res. 2002;12:539-47. [PubMed]
62. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340-6. [PubMed]
63. Miracco C, Santopietro R, Biagioli M, Lazzi S, Nyongo A, Vatti R, Luzi P. Different patterns of cell proliferation and death and oncogene expression in cutaneous malignant melanoma. J Cutan Pathol. 1998;25:244-51. [PubMed]
© 2009 Dermatology Online Journal