The post-thrombotic syndrome - a condition to prevent
Published Web Location
https://doi.org/10.5070/D36xc40751Main Content
The post-thrombotic syndrome - a condition to prevent
Delphine Pirard MD1, Bernard Bellens MD2, Pierre Vereecken MD1,3,4
Dermatology Online Journal 14 (3): 13
1. Department of Dermatology, Erasme hospital, Université Libre de Bruxelles (U.L.B.) Brussels, Belgium 2. Department of Vascular
Surgery, CHU-Brugmann, Brussels, Belgium 3. Department of Dermatology, CHU-Brugmann, Brussels, Belgium. 4. Department of Medical
Oncology, Jules Bordet Institute, Brussels, Belgium. pierre.vereecken@chu-brugmann.beAbstract
The incidence of the post-thrombotic syndrome (PTS) is increasing along with the incidence of deep vein thrombosis (DVT). The overall frequency of PTS ranges from 20 percent to 50 percent of DVT patients; severe PTS, which includes leg ulcers, occurs in a quarter of cases. Because of its severity and chronicity, PTS is associated with great morbidity and cost. Its diagnosis is primarily based on the presence of typical symptoms and signs, but objective evidence of venous valvular reflux can help to confirm the diagnosis. Because therapeutic options for PTS are extremely limited and results are often disappointing, prevention, recognition of clinical signs or complications, and early treatment remain the keys to reducing its morbidity. The prevention of DVT recurrence by anticoagulation and use of graduated compression stockings is likely to reduce the risk of PTS. There is no proven role for thrombolysis in preventing PTS.
Introduction
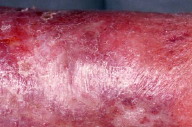
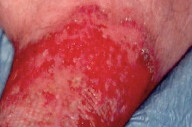
Despite the widespread use of thromboprophylaxis, the incidence of deep venous thrombosis (DVT) has been increasing [1]. The post-thrombotic syndrome (PTS) develops in 20 to 50 percent of patients within 1 to 2 years of DVT and among these, 25 percent suffer from severe PTS [2]. The pathophysiology of PTS is incompletely understood but it is thought that residual venous abnormalities, consisting of persistent truncular obstruction, valvular incompetence, reflux in deep veins, and failure of the calf venous pump lead to increased venous and capillary pressure [3, 4]. The increased pressure causes edema and rupture of small superficial veins, subcutaneous hemorrhage, and deposition of hemosiderin (stasis pigmentation). Edema remains intermittent as long as the lymphatic network withstands the excess workload. Thereafter, a lymphatic microangiopathy develops, and edema of mixed origin occurs [5]. Subsequent subcutaneous fibrosis and cutaneous atrophy lead to the condition termed lipodermatosclerosis (Fig. 1). This sclerosing panniculitis is thought to be a maladaptive healing process secondary to microvascular changes [6, 7].
 |
| Figure 3 |
|---|
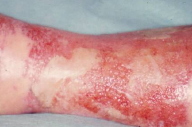
| Figure 3. Image of a bullous erysipela: acute inflammatory episodes are common consequences of lymphoedema because of an increased susceptibility to bacterial and fungal infections. |
The skin is atrophic, shiny, hyperpigmented, and fixed to the firm subcutaneous tissue. This results in the "inverted champagne bottle" appearance (Fig. 2). Lipodermatosclerosis can also develop in patients with chronic venous stasis without prior history of DVT. Patients complain of pain, heaviness, cramps, itching, or tingling in the affected limb. Early complications include stasis ulceration, acute inflammatory episodes (often mistaken for cellulitis), and fungal or bacterial infections. The recurrent inflammation and infection can worsen venous and lymphatic damage and subsequent edema (Fig. 3). The progression of lipodermatosclerosis results in disfigurement, decrease in quality-of-life, immobility, elevated risk of recurrent DVT, and increased societal burden [8]. A less frequent but more severe complication of chronic lymphedema is lymphangiosarcoma (Stewart-Treves syndrome), although this tumor generally occurs in lymphedematous limbs subsequent to lymphadenectomy. This aggressive sarcoma presents with violaceous, cutaneous papules and plaques in areas of lymphedema and has a 3 year median survival [9]. These clinical symptoms and signs are summarized in Table 1.
Diagnosis of PTS
Although the diagnosis of PTS is essentially based on the development of the above-mentioned clinical manifestations in patients with a history of DVT, it may be helpful to confirm that the edema of the lower leg is of venous origin. Doppler ultrasound, magnetic resonance imaging, and lymphoscintigraphy are helpful in confirming the diagnosis [10]. Objective testing may also be essential when recurrence of ipsilateral DVT is suspected in PTS patients with new or acute episodes of leg pain and swelling. In this case, compression ultrasonography results may be altered by persistent abnormalities after the initial DVT [11], but the D-dimer testing might be useful. This test detects the cross-linked fibrin degradation fragment D-dimer. Elevations in this fragment are seen in primary and secondary fibrinolysis, as a result of thrombotic disease.
Clinical scales have been developed to classify DVT patients as having PTS. The Villalta scale [12] grades the severity, from 0 to 3 of five symptoms (pain, cramps, heaviness, pruritus and paresthesia) and six signs (edema, skin indutation, hyperpigmentation, venous ectasia, redness and pain during calf compression); a summed total score of ≥ 5 indicates PTS, and > 14 or presence of ulcer indicates severe PTS. In the Grinsberg measure [13], PTS is defined by the presence of daily leg pain and swelling for 1 month, occurring 6 months or more after DVT, made worse by standing/walking, and relieved by rest/leg elevation. This last measure seems to identify more severe disease [14].
Management of PTS patients
Available treatments for established PTS are limited: elevation, lymphatic massage, exercise, skin care, and compression therapy. Compression bandages are useful in the acute phase of leg swelling or venous ulceration; compression stockings are valuable in the maintenance phase [15, 16]. In lipodermatosclerosis, class I compression (18 to 26 mm Hg) seem to be as effective as class II (26 to 36 mm Hg) compression for elimination of dermal edema. However, only class II compression stockings have been shown to prevent secondary DVT [17, 18]. Greater benefits are to be expected if compliance with compression therapy is monitored through ambulatory care programs. Skin care is also of the utmost importance. Liberal use of emollients is recommended and topical corticosteroids should be added for inflammatory dermatitis. Because atrophy is often a prominent feature, a return to bland emollients should be encouraged once the dermatitis has resolved. The addition of 2-5 percent salicylic acid can treat excessive hyperkeratosis. When oozing and crusting or verrucous changes are present, compresses with antiseptic drying agents, such as aluminium acetate (Burow's solution) or aluminium sulfate and calcium acetate (Domboro's solution), are important to dry and prevent overgrowth of bacteria in deep crevices. Control of bacterial or fungal infection is essential. Oral antibiotics should be started rapidly if bacterial infection is suspected. Stanozolol, known to be fibrinolytic, was reported as effective in improving lipodermatosclerosis, but there is no proven role for veno-active medications or diuretics in the management of PTS [19]. Severe PTS can be improved with long-term use of an intermittent compression extremity pump [20].
Discussion
The best way to manage PTS is by prevention: early identification of risk factors, prevention of DVT, and aggressive management of DVT [21]. In prospective studies, the only clearly identified factors that predict the development of PTS after DVT are recurrent, ipsilateral DVT and inadequate oral anticoagulation after DVT [22]. Neither the severity nor the localization of the thrombus seems to influence the subsequent development of PTS. However, iliofemoral DVT seems to be associated with the most severe PTS morbidity [23]. The prevention of DVT, or thromboprophylaxis, is important in high-risk patients, as defined in regularly updated consensus guidelines [24]. The treatment of choice of uncomplicated DVT is anticoagulation, which is highly effective in preventing recurrent, especially ipsilateral, venous thromboembolism.
The currently recommended approach is to start heparin and vitamin K antagonists (VKAs) together at the time of diagnosis, and to discontinue heparin when the international normalized ratio (INR) is stable and > 2.0; this usually occurs after 5 to 7 days of heparin therapy. The subsequent doses should be adjusted to maintain the INR at a target of 2.5 (range, 2.0 to 3.0) [25]. The recommended duration of anticoagulation is at least 3 months of treatment after proximal DVT and may be sufficient for patients with temporary risk factors and a low risk of recurrence. For patients with idiopathic VTE or permanent risk factors at least 6 months anticoagulation is recommended [26] In addition, DVT patients should use antithrombosis class I compression stockings and practice anti-stasis exercises. In some patients, intermittent external pneumatic compression may be required. The regular use of compression stockings should be continued for at least 2 years after the acute DVT event and longer if swelling persists [27]. Recent studies show that, for ilio-femoral thrombus, endovascular catheter-directed thrombolysis techniques with pharmacologic thrombolytic agents, used alone or in combination with mechanical thrombectomy devices might allow the preservation of venous valve function. Nevertheless, there is no definitive evidence that these techniques will decrease the incidence of PTS compared with the use of conventional anticoagulation [28].
Conclusion
PTS leads to chronic and severe morbidity. Its best treatment remains prevention. In established DVT patients, anticoagulation, compression, exercise, and skin care measures are needed in order to prevent DVT recurrence and long-term sequellae such as PTS.
References
1. Kahn SR, Ginsberg JF. Relationship between deep venous thrombosis and the post-thrombotic syndrome. Arch Intern Med 2004; 164: 17-26. PubMed2. Kahn SR, Ginsberg JF. The post-thrombotic syndrome: current knowledge, controversies, and directions for future research. Blood Rev 2002; 16: 155-65. PubMed
3. Hopkins NF, Wolfe JH,. ABC of vascular diseases: deep venous insufficiency and occlusion. BMJ 1992; 304: 107-10. PubMed
4. van Haarst EP, Liasis N, van Ramshorst B, Moll FL. The development of valvular incompetence after deep vein thrombosis: a 7 year follow-up study with dupplex scanning. Eur J Vasc Endovasc Sur 1996; 12: 295-9. PubMed
5. Bollinger A, Isenring G, Franzeck UK. Lymphatic microangiopathy: a complication of severe chronic venous incompetence (CVI). Lymphology 1982; 15: 60-5. PubMed
6. Jorizzo JL, White WL, Zanolli MD et al. Sclerosing panniculitis: a clinicopathologic assessment. Arch dermatol 1991; 127: 554-8. PubMed
7. Bruce AJ, Bennett DD, Lohse CM et al. lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46: 187-92. PubMed
8. Kahn SR, Hirsch A, Shrier I. Effect of post-thrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162: 1144-8. PubMed
9. Sibaud V, Toussaint P, Labbe L et al. Nodules violacés sur lymphoedème. Ann Dermatol Venereol 2000; 127: 631-2. PubMed
10. Wheatley DC, Wastie ML, Whitaker SC et al. Limphoscintigraphy and colour doppler sonography in the assessment of leg oedema of unknown cause. Br J Radiol 1996; 69: 1117-24. PubMed
11. Bates SM, Grand'Maison A, Johnston M et al. A latex D-dimer reliably excludes venous thromboembolism. Arch Intern Med 2001; 161: 447-53. PubMed
12. Villata S, Bagatella P, Piccioli A et al. Assessment of validity and reproducibillity of a clinical scale for thr post-thrombotic syndrome. Haemostasis 1994; 24: 158a (abstract).
13. Ginsberg JS, Gent M, Turkstra F, et al. Post-thrombotic syndrome after hip or knee arthroplasty: a cross-sectional study. Arch Intern Med 2000; 160: 669-72. PubMed
14. Kahn SR, Desmarais S, Ducruet T, Arsenault L, Ginsberg JS. Comparison of the Villalta and Ginsberg clinical scales to diagnose the post-thrombotic syndrome: correlation with patient-reported disease burden and venous valvular reflux.J Thromb Haemost 2006 Apr;4(4):907-8. PubMed
15. Eliska O, Eliskova M. Are peripheral lymphatics damaged by high pressure manual massage? Lymphology 1995; 28: 21-30. PubMed
16. Stoberl C. Compression therapy in post-thrombotic syndrome. Wien Med Wochenschr 1994; 144: 233-7. PubMed
17. Gniadecka M, Karlsmark T, Bertram A. Removal of dermal edema with class I and II compression stockings in patients with lipodermatosclerosis. J Am Acad Dermatol 1998; 39: 966-70. PubMed
18. McCollum C. Avoiding the consequences of deep vein thrombosis: elevation and compression are important and too often forgotten. Br Med J 1998; 12: 696. PubMed
19. Helfman T, Falanga V. Stanozolol as a novel therapeutic agent in dermatology. J Am Acad Dermatol 1995; 33: 254-8. PubMed
20. Ginsberg JS, MagierD, MacKinnon B et al. Intermittent compression units for severe post-phlebitic syndrome: a randomized cross-over study. CMAJ 1999; 160: 1303-6. PubMed
21. Bernardi E, Prandoni P. The post-thrombotic syndrome. Curr Opin Pulm Med 2000; 6: 335-42. PubMed
22. Prandoni P, Villalta S, Bagatella P, et al. The clinical course of deep vein thrombosis: prospective long-term follow-up of 528 symptomatic patients. Haematologica 1997; 82: 423-8. PubMed
23. Comerota AJ. Quality-of-life improvement using thrombolytic therapy for ilio-femoral deep venous thrombosis. Rev Cardiovasc Med 2002; 3(suppl 2): S61-7. PubMed
24. Geerts WH. Prevention of venous thromboembolism in high-risk patients. Hematology Am Soc Hematol Educ Program 2006;462-6. PubMed
25.Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE.Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest. 2004 ;126:401S-428S. PubMed
26. Baglin TP, Keeling DM, Watson HG.Guidelines on oral anticoagulation (warfarin): third edition--2005 update.Br J Haematol. 2006 Feb;132(3):277-85. PubMed
27. Brandjes DP, Buller HR, Heijboer H et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997; 349: 759-62. PubMed
28. Sharafuddin MJ, Sun S, Hoballah JJ, et al. Endovascular management of venous thrombotic and occlusive disease of the lower extremities. J Vasc Interv Radiol 2003; 14: 405-23. PubMed
© 2008 Dermatology Online Journal