Association between solitary keratoacanthoma and cigarette smoking: A case-control study
Published Web Location
https://doi.org/10.5070/D32wt2t8hmMain Content
Association between solitary keratoacanthoma and cigarette smoking: A case-control study
Hélio Amante Miot PhD1, Luciane Donida Bartoli Miot MD1, Ana Laura Bastos da Costa2, Cristiane Yuri Matsuo2, Hamilton Ometto Stolf PhD1, Mariângela Esther Alencar Marques PhD3
Dermatology Online Journal 12 (2): 2
1. Dermatology Department of Unesp Medical School, Botucatu SP, Brazil. heliomiot@fmb.unesp.br2. Unesp Medical School, Botucatu - SP - Brazil
3. Pathology Department of Unesp Medical School, Botucatu - SP - Brazil
Abstract
Solitary keratoacanthoma (KA) is a common benign epithelial tumor of the skin characterized by rapid growth and a tendency toward spontaneous regression. The exact etiology and classification of KA are a matter of debate. Smokers also seem to be more affected than persons who never smoke. The objective of this study was to evaluate the association between solitary KA and smoking habit. A case-control study involving 78 patients diagnosed with KA and 199 controls from the related community was performed to evaluate the association between cigarette smoking and KA. A higher smoking prevalence was noted in cases (69.2 %) than controls (21.6 %) and the odds ratio adjusted for sex and age was 9.1 (95 % CI 4.9 to 17.1, p< 0.01). The mean tumoral diameter at surgery and the site of involvement was not statistically related to smoking. These findings suggest that cigarette smoking is associated with the development of KA.
Introduction
Keratoacanthoma (KA) is a common epithelial tumor of the skin characterized by rapid growth, histopathologic features similar to those of cutaneous squamous cell carcinoma, and a tendency toward spontaneous regression in absence of treatment [1].
The exact nosology and classification of KA are a matter of debate. Some authors regard KA as a benign cutaneous tumor that is the prototype of the "pseudomalignant" tumors of the skin, whereas others maintain that it truly represents a malignant neoplasm and should be regarded as a peculiar variant of cutaneous squamous cell carcinoma [2, 3, 4].
KA most often appears on sun-exposed regions of light-complected persons; a majority of tumors occur on the face, forearms and hands [5]. The peak of incidence of the KA occurs between the ages 50-69, however it is reported in all age groups, being rare in younger than 20 years; its incidence increases with age. Studies on gender distribution reveal that both sexes are affected equally or there is a slight predilection for males [6].
There are three distinct clinical stages in the natural history of the KA, proliferative, mature, and involutional. Some lesions persist for a year or more although the entire process from origin to spontaneous resolution usually takes about 4-6 months [1, 7].
Keratoacanthomas have many morphological forms or syndromic types, however solitary KA is the most common type [7, 8].
The etiology of the KA is unknown, and it is assumed to derive from hair follicles in humans. Keratin analysis of KA suggests both the characteristics of follicular differentiation and of a squamous cell carcinoma [7]. Chromossomic aberrations were described in KA series, and many of the genetic patterns differ from those of squamous cell carcinoma, but a single pattern characterizing KA was not yet identified [9].
It is possible that many etiological agents are involved in the formation of KA in man and that not all KA are produced by the same agent or groups of agents. Some related etiological factors are as follows: ultraviolet rays, chemical carcinogens (tar, pitch, mineral oil), trauma, genetic factors, infections, human papilloma-virus (HPV), and immunosuppression. In addition, KA has been observed in patients affected by a variety of skin diseases, including psoriasis, discoid lupus erythematosus, lichen planus, atopic dermatitis, herpes zoster, acne conglobata, radiodermatitis, scars, pemphigus foliaceus, xeroderma pigmentosum, Muir-Torre syndrome, and others [1, 10, 11]. Cigarette smoking is suggested as an environmental trigger that induces the onset of KA in predisposed individuals [1, 10, 12, 13]. This study aims to evaluate the association between cigarette smoking and solitary KA.
Methods
A case-control study was performed. Epidemiological information on KA and smoking habits were obtained with a standard questionnaire. Cases with clinical and histopathological diagnosis of KA were selected from the dermatology clinic of Hospital das Clínicas at Unesp Medical School. Immunosuppressed patients with KA were not included to this study. Two or three controls were selected for each case from spouses, close relatives and household members; they were matched with cases according to sex, age, and city of origin [14]. Cases were personally interviewed after their consultation and controls were individually contacted by telephone.
Patients defined as smokers (current or former) were those who had been smoking at least 4 cigarettes per day for at least 5 years. The odds ratio was adjusted for possible confounding factors, age and sex using unconditional multiple logistic regression [14]. Population attributable risk was calculated using the adjusted odds ratio, and smoking prevalence in the control group represented prevalence in the population [15].
The research was approved by the research council of the Dermatology Department of Unesp Medical School. Data was recorded and analyzed with Bioestat 2.0™, considering a significance of p< 0.05 [16].
Results
Of the 133 patients initially identified, there were 78 who qualified for inclusion; they had solitary-type KAs that were not attributed to immunosuppression and their controls could be contacted by telephone. The 199 controls were representative in comparison to cases for gender, age (See Table I) and cities of origin. All patients and controls came from cities with an ultraviolet index of 5. Among the cases, there were 1.2 males to 1 female, but there was no statistical difference in this rate (χ², p> 0.05). Smoking was more prevalent in men from cases and it also occurred in controls (See Table II). Smoking prevalence was significantly higher for cases at ages up to age 40.
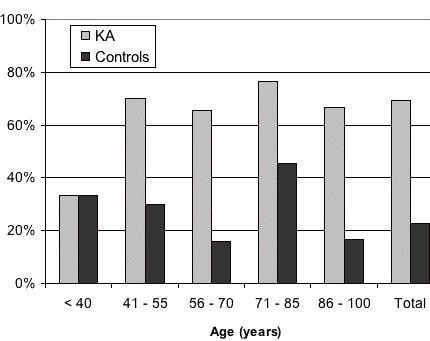 |
| Figure 1 |
|---|
| Figure 1: Smoking prevalence according to age distribution. (χ² p< 0.05). |
The mean age for disease development was 64.9 years, with no differences according to sex or smoking exposure. The mean tumoral diameter at surgery was 1.3 cm, although smoking was not statistically associated to size (Table II). Most KA cases had started smoking at an early age (mean 17.5 years) and presented an average consumption of 21.5 cigarettes per day. Smoking prevalence between cases was higher in men (80.1%) than women (55.6%) (χ², p< 0.05) (Table II). All KA patients defined as smokers started smoking prior to developing the tumor, with a mean interval of 50.3 ± 15.2 years, but there was no relationship between the number of cigarettes smoked or accumulated smoking exposure until disease development (Pearson, p> 0.05).
Former smokers represented 38.9 percent of smokers among cases, i.e., those who gave up smoking more than 5 years before the onset of KA. The mean duration of smoking for former smokers was 24.7 years. Only two patients had 2 KA's: a male smoker and a female nonsmoker, and both were affected on the face and upper arm; only the first KA diagnosed in both subjects was considered for this study.
KA affected mostly the upper arms (44.9%), followed by head and neck, and legs (Table III). Topographical distribution of KA was not related to smoking or age (Fisher, p> 0.05), but the occurrence in legs was significantly more prevalent in women (Fisher, p< 0.05). There was higher smoking prevalence in cases (69.2%) compared to controls (22.6 %) (Table IV) and the calculated odds ratio after adjustment for sex and age was 9.1 (CI 95 % 4.9 to 17.1, multiple logistic regression, p< 0.01) for smokers. Compared to never smokers, current smokers represent 57.9 percent and former smokers 46.7 percent of cases. Population attributable risk was estimated as 64.8 percent (CI 95% 47.9 to 77.8%).
Discussion
The risk of cutaneous malignancies related to smoking is relatively unknown, however associations have been described with basal cell carcinoma, squamous cell carcinoma, malignant melanoma and KA [17, 18].
Chemical tumorigenesis has been impressively documented with regard to KA in a number of animal models by painting of skin with tar derivatives. A comparative study of carcinogenic effects produced on 100 mice by painting with two types of tobacco (black and light) showed that 80 percent of the 176 induced tumors were KA [19].
In humans, a study of 250 KA arising in 238 patients during a 6-year period showed a significantly increased incidence of these tumors in pitch and tar workers over matched controls. At this study it was pointed out that 66.5 percent of cases were tobacco smokers [10].
The effects of smoking on the development of skin cancer can potentially be explained in a number of different ways. First, tobacco smoke may act as a skin carcinogen, either directly on the skin or through a systemic carcinogenic effect. The association between several malignancies at sites remote from direct smoke contact, such as the bladder, lung, stomach, pancreas, and cervix, suggests systemic carcinogenic effects of smoking. Even passive smokers could be affected from tobacco carcinogenesis [20], but our study could not determine the role of passive smoking in KA development.
Tobacco smoke also contains several classes of compounds with demonstrated carcinogenic or cocarcinogenic activity, including nitrosamines, polycyclic aromatic hydrocarbons, aromatic amines, unsaturated aldehydes, and phenolic compounds. Among these, benzo[a]pyrene, the prototype compound of polycyclic aromatic hydrocarbons, is metabolically activated to the ultimate carcinogen benzo[a]pyrene diolepoxide, which may form DNA adducts; this is generally thought to be the basis of its carcinogenic effect. Several mouse studies have shown the binding of benzo[a]pyrene diolepoxide to DNA to correlate with the initiation of skin carcinogenesis [17]. Numerous reports have pointed out the importance of the p53 tumor suppressor gene in the process of carcinogenesis. Also the induction of specific mutations in the p53 gene could be a way by which tobacco smoke exerts its carcinogenic effect on human skin [17].
In addition, immunosuppression caused by nicotine consumption may contribute to the pathogenesis of HPV infections, malignant melanoma, and epithelial tumors of the skin and neighboring mucous membranes [18].
Studies show also an association between HPV infection and KA. For other malignancies, such as cervical carcinoma, smoking acts as a cofactor for the development of cancer [21, 22]. Further studies are needed to investigate the association of HPV and smoking as cofactors in KA development.
The median age that smoking commenced in our study was very early; this may provide a longer cumulative smoking exposure and more opportunity to trigger disease in predisposed individuals. All cases defined as smokers developed the disease many years after they had started to smoke. The age of KA onset, male to female rate, and topographic distribution (photoexposed areas) of lesions were in accordance with literature. The significant compromise in female legs, was also observed by other authors and could be explained by a greater sun exposure at this site than occurs in men [5].
Smoking is the most important environmental risk in global public health. Reductions in smoking rates would have a direct favorable impact on more than 50 diseases. It is hard to estimate the real prevalence of smoking in the Brazilian population. Smoking habits are influenced by sex, education, age, and cultural, familial and social aspects. The choice of more than one control for each case was aimed at increasing statistical power and lowering sampling errors to reduce the chances of selection bias, because smoking has so many external influences [14]. About one third of the world adult population smokes. Males still represent the larger proportion of smokers, and prevalence of smoking is still increasing among women and also in developing countries [23]. A recent survey among employees of Hospital das Clínicas from Unesp medical school indicated a general smoking prevalence of 21.3 percent, and workers more than 35 years old accounted for 25.7 percent of smokers. In 1989 26 percent of Brazilian women and 40 percent of men over age 15 smoked. In other case-control studies in Brazil, smoking prevalence ranged from 25 to 34 percent [24, 25].
From case-control studies, we can also estimate the percentage of population attributable risk. Regarding the prevalence of smoking in the population and the role of smoking in KA pathogenesis, 64.8 percent of KA cases could have been influenced by smoking and might have been prevented if exposure to smoking did not exist.
This study did not evaluate the consumption of caffeine, alcohol, analgesics, or hypnotics, which have higher use among smokers and could also play a role in KA pathogenesis. All cases were fair-skinned persons, and a precise racial or phototype estimation of controls by telephone interviews could not be done. However, skin type was not related to risk of smoking in the literature. Cumulative ultraviolet or chemical exposure from professional exposure and outdoor activities could not be isolated because of imprecise data from participants and more than 40 percent of cases were reported to be retired at the interview. Nevertheless, the strong association between smoking and KA found in this study (OR of 9.1), reduces the possibility of this being a chance result or confounding interference; it suggests that smoking can represent an independent risk factor for KA.
Conclusions
This study detected a statistical association between cigarette smoking and KA. The higher smoking prevalence in KA patients suggests a relationship with disease development and these findings can also reinforce an opportunity for health education against smoking. New studies are needed in order to determine how much population giving up smoking would affect the KA incidence rates and its impact on the clinical course of the disease, as well to elucidate the specific role of smoking in tumorigenesis.
Acknowledgments: Dr. George Barros Leal who gently reviewed this paper.
References
1. Schwartz RA. Keratoacanthoma. J Am Acad Dermatol 1994; 30(1):1-19. PubMed2. Beham A, Regauer S, Soyer HP, Beham-Schmid C. Keratoacanthoma: a clinically distinct variant of well differentiated squamous cell carcinoma. Adv Anat Pathol 1998; 5(5):269-80. PubMed
3. Warner DM. Solitary Keratoacanthoma (Squamous Cell Carcinoma): Surgical Management. Internat J Dermatol 1995; 34(1):17-9. PubMed
4. Hodak E, Jones RE, Ackerman AB. Solitary Keratoacanthoma Is a Squamous-Cell Carcinoma: Three Examples with Metastases. Am J Dermatopathol 1993; 15(4): 332-42. PubMed
5. Schwartz RA. The Keratoacanthoma: A Review. J Surg Oncol 1979; 12:305-17. PubMed
6. Cerroni L, Kerl H. Chapter 83: Keratoacanthoma. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick's dermatology in general medicine, 6th edition. Boston: McGraw-Hill, 2003: 760-7.
7. Schwartz RA. Keratoacanthoma: A Clinico-Pathologic Enigma. Dermatol Surg 2004; 30:326-33. PubMed
8. Seifert A, Nasemann T. Das keratoakanthom und seine klinischen varianten Hautarzt 1989; 40:189-202. PubMed
9. Clausen OPF, Beigi M, Bolund L, Kolvraa S, Gjersvik PJ, Mork G, De Angelis PM. Keratoacanthomas frequently show chromosomal aberrations as assessed by comparative genomic hybridization. J Invest Dermatol 2002; 119:1367-72. PubMed
10. Ghadially FN, Barton BW, Kerridge BW. The etiology of keratoacanthoma. Cancer 1963; 16:603-11. PubMed
11. Sullivan JJ. Keratoacanthoma. The Australian experience. Australas J Dermatol 1997; 38:S36-S39. PubMed
12. El-Hakim IE, Uthman MAE. Squamous cell carcinoma and keratoacanthoma of the lower lip associated with "Goza" and "Shisha" smoking.Internat J Dermatol 1999; 38(2):108-10. PubMed
13. Smith JB, Fenske NA. Cutaneous manifestations and consequences of smoking. J Am Acad Dermatol 1996; 34:717-32. PubMed
14. Hennekens CH, Buring JE. Epidemiology in Medicine. 1st edition, Boston (MS): Little Brown and Co; 1987.
15. Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimation of population attributable risk for multiple factors using case-control data. Am J Epidemiol 1985; 122:904-14. PubMed
16. Ayres M, Ayres Jr M, Ayres DL, dos Santos AS: Bioestat: 2.0 Aplicações Estatísticas nas áreas das ciências biológicas e médicas. Belém (PA): Sociedade Civil Mamirauá e MCT – CNPq; 2000.
17. De Hertog SAE, Wensveen CAH, Bastiaens MT, Kielich CJ, Berkhout MJP, Westendorp RGJ, Vermeer BJ, Bouwes BJN. Relation between smoking and skin cancer. J Clin Oncol 2001; 19(1):231-8. PubMed
18. Krug M, Wünsche A, Blum A. Tabakabhängigkeit und die folgen auf die haut. Hautartz 2004; 55(3):301-15. PubMed
19. Munoz N, Correa P, Bock FG. Comparative carcinogenic effect of two types of tobacco. Cancer1968; 21:376-89. PubMed
20. Kawachi I. More evidence on the risks of passive smoking. BMJ 2005; 330:265-6. PubMed
21. Hsi ED, Svoboda-Newman SM, Stern RA, Nickoloff BJ, Frank TS. Detection of human papillomavirus DNA in keratoacanthomas by polymerase chain reaction. Am J Dermatopathol. 1997; 19(1):10-5. PubMed
22. Castellsagué X, Munõz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis-role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr 2003; 31:20-8.
23. Achutti A, Menezes AMB: Epidemiologia do tabagismo. In: Achutti A. Guia Nacional de Prevenção e Tratamento do Tabagismo. Rio de Janeiro (RJ): Vitrô Comunicação & Editora; 2001. p.9-27.
24. Menezes AMB, Horta BL, Oliveira ALB, Kaufmann RAC, et al: Risco de câncer de pulmão, laringe e esôfago atribuível ao fumo. Rev Saude Publica 2002; 36:129-34.
25. Ministério da Saúde. Instituto Nacional de Alimentação e Nutrição (INAN). PNSN: estatísticas sobre hábitos de fumo no Brasil. Brasília (DF); 1989.
© 2006 Dermatology Online Journal

