Coexisting basal cell carcinoma and metastatic small cell carcinoma of lung
Published Web Location
https://doi.org/10.5070/D3418498mfMain Content
Coexisting basal cell carcinoma and metastatic small cell carcinoma of lung
Shylashree Chikkamuniyappa MD
Dermatology Online Journal 10 (1): 18
Department of Pathology, University of Texas Health Science Center at San Antonio. chikkamuniya@uthscsa.edu
Abstract
The coexistence of basal cell carcinoma with a cutaneous metastasis is rare; it is most frequently associated with lung cancer. We report an unusual case of contralateral metachronous metastasis of small-cell carcinoma of the lung to a site of basal cell carcinoma.
Introduction
The coexistence of basal cell carcinoma with a cutaneous metastasis is rare. The most frequent primary tumor in man causing a cutaneous metastasis is lung cancer. Cutaneous metastases are found in up to 4 percent of patients with carcinoma of the lung. The first case of skin metastasis of small cell carcinoma of lung, described in 1983, was confirmed by electron microscopy [1]. Our case represents a metachronous metastasis with basal cell carcinoma, primary tumor of that was confirmed by immunohistochemistry.
The term metachronous metastasis is used when the cutaneous metastasis develops several months to years after the primary malignancy has been diagnosed. Metastasis tends to occur most frequently on the cutaneous surfaces near the site of the primary tumor. Our case is unique in two aspects. First, it is represents a metachronous metastasis of the lung co-existing with a basal cell carcinoma and second, it occurred on the opposite side of the primary tumor.
Clinical summary
A 59-year-old male presented to the Dermatology clinic with a rapidly growing skin mass of 2-weeks duration on the right shoulder. Shave biopsy of the lesion was diagnosed as basal cell carcinoma. Since the mass was huge, a wide resection was performed in the OR by the surgery team. On physical examination, there was no lymphadenopathy. The patient had been diagnosed 9 months earlier with small cell carcinoma of the lung, presenting as a left hilar mass with mediastinal lymphadenopathy. He had received radiation, with two more treatments remaining, and had refused chemotherapy. He is also a hypertensive and diabetic with a history of von-Willebrand disease.
|
|
| Figure 1 | Figure 2 |
|---|---|
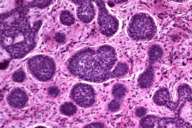
| Low power magnification of basal cell carcinoma (H&E,10X) | |
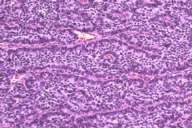
The specimen received showed a scar with large central ulceration. The tumor was 5.5 cm in greatest dimension and was partly necrotic. Histological examination showed two distinct growth patterns. One was a neoplastic proliferation of atypical basaloid cells with scant cytoplasm and hyperchromatic, pleomorphic nuclei. These sheets of cells showed peripheral palisading and adjacent fibrous stroma, which was consistent with basal cell carcinoma. The second pattern showed cords and trabeculae of small neoplastic cells with scant cytoplasm and vesicular nuclei with a neuroendocrine (salt and pepper chromatin) appearance.
The differential diagnoses considered clinically, along with a metastatic small cell carcinoma of the lung, were squamous cell carcinoma, basal cell carcinoma, amelanotic melanoma, carcinoid tumor, Merkel cell carcinoma, neuroendocrine carcinoma, malignant fibrous histiocytoma, atypical fibroxanthoma, and dermatofibrosarcoma protuberans. Cytokeratin 20 was negative, ruling out Merkel cell carcinoma.
|
| Figure 3 |
|---|
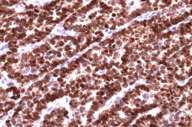
| Immunostain with TTF-1 (10X) |
Immunohistochemical stain with TTF-1 (thyroid transcription factor) was positive confirming that it was a lung primary.
|
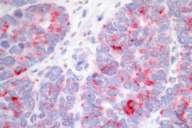
|
| Figure 4 | Figure 5 |
|---|---|
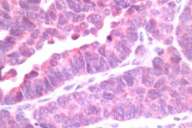
| Immunostain with neuron-specific enolase (Fig. 4) (40 ×) | |
| Immunostain with chromogranin (Fig. 5) (40 ×) | |
The neuroendocrine markers of neuron-specific enolase (NSE) and chromogranin were positive. The combination of TTF-1, NSE and chromogranin positivity shows that it is a small cell carcinoma of lung. Carcinoid tumors are typically TTF-1 negative and show positivity with NSE and chromogranin. The histologic pattern ruled out the remaining differential diagnosis.
Discussion
Basal cell carcinoma is the most common of the cutaneous malignancies, accounting for 65-75 percent of all skin cancers [2]. These carcinomas are usually slow-growing, locally aggressive tumors that rarely metastasize. Basal cell carcinomas have been described to occur synchronously with squamous cell carcinomas and Merkel cell carcinoma.
Cutaneous metastasis of the lung occurs in up to 4 percent. The histological pattern is squamous cell carcinoma in 40 percent, adenocarcinoma in 20 percent, and undifferentiated carcinoma in 40 percent [3]. One case of bronchiolar, one case of mucoepidermoid bronchial carcinoma, and pleural mesotheliomas have also been reported [4, 5]. The largest study of pulmonary tumors (579) showed a 2.8 percent incidence of cutaneous metastasis [6].
Small cell carcinoma originates from the bronchi and is usually fatal; most patients die within 1 year of presentation. Untreated, patients survive for only 1-3 months after diagnosis. Survival is short even when patients are treated and metastases occur in a very short time. The mainstay of treatment is radiation and chemotherapy. Despite therapy most patients develop metastases; the mean survival period is 8-15 months [7]. The disease most frequently metastasizes to central nervous system, bone marrow, suprarenal glands, and lymph nodes of the neck. Small cell cancer may be accompanied by paraneoplastic syndromes, vena cava syndrome, and compressions to the spinal cord. Skin metastasis are rarely described. The skin of the chest and abdomen is the usual site, but lung cancer metastases also show a predilection to the back [8]. Other sites like umbilicus, lips, tongue, and foot have been described. The development of cutaneous metastasis portends a grave prognosis. This is the first case of a basal cell carcinoma associated with metastatic small cell carcinoma.
References
1. Skin metastases from small-cell carcinoma of the lung. J Dermatol Surg Oncol. 1983 Jun;9(6):451-4. PubMed2. Malone JP, Fedok FG, Belchis DA, Maloney ME. Basal cell carcinoma metastatic to the parotid: report of a new case and review of the literature. Ear Nose Throat J. 2000 Jul;79(7):511-5, 518-9. PubMed
3. De Argila D. Bureo JC. Marquez FL. Pimentel JJ. Small-cell carcinoma of the lung presenting as a cutaneous metastasis of the lip mimicking a Merkel cell carcinoma. Clin Exp Dermatol. 1999 May;24(3):170-2. PubMed
4. Brownstein MH, Helwig EB. Metastatic tumors of skin. Cancer. 1965;18:907-915
5. Dutt PL, Baxter JW, O'Malley FP et al. Distant cutaneous metastasis of pleural malignant mesothelioma. J Cutan Pathol. 1992 Dec;19(6):490-5. PubMed
6. Hidaka T, Ishii Y, Kitamura S. Clinical features of skin metastasis from lung cancer. Intern Med. 1996 Jun;35(6):459-62. PubMed
7. Niiranen A. Long-term survival in small cell carcinoma of the lung. Eur J Cancer Clin Oncol. 1988 Apr;24(4):749-52. PubMed
8. Brady LW, O'Neill EA, Farber SH. Unusual sites of metastases. Semin Oncol. 1977 Mar;4(1):59-64. PubMed
© 2004 Dermatology Online Journal

